The moral life of epinephrine in the United States
—
Abstract
Introduction
The first time it occurred to me to think of epinephrine auto-injectors as having a ‘moral life’, I was sitting in a middle school auditorium in a well-to-do suburb in the United States in 2014. A well-known allergist from a major regional teaching hospital was giving a lecture to a group of school nurses. Echoing current medical consensus (Sicherer and Sampson 2010), he considered epinephrine auto-injectors to be the only treatment for anaphylaxis, a serious allergic reaction often experienced by people with food allergies. During his talk, the allergist repeated, ‘anaphylaxis is epinephrine’. The implication of his statement was that the only appropriate technical response to an allergic reaction was interventional treatment with epinephrine, provided to patients as expensive, branded commodities called epinephrine auto-injectors. For many physicians, prescribing epinephrine for acute reactions is the extent of the biomedical response to the condition, despite the many lifestyle challenges that it poses and the many steps that can be taken to prevent a reaction in the first place.
The response of a nurse sitting a few seats away from me suggested that the typical protocol governing the use of epinephrine is a moral as well as a technical issue. She raised her hand at the end of the talk to explain to the speaker and the audience that she cared deeply about the kids at her school. The responsibility for keeping ‘her kids’ safe and healthy at a moment when growing numbers of them had food allergy diagnoses weighed heavily on her. Due to her role as a nurse, only she was allowed to use life-saving medications like epinephrine to guard against the deaths of children from anaphylaxis in her school. This responsibility weighed heavily upon her. As she spoke, other nurses nodded and mumbled in sympathy. All of them care for a growing number of children with food allergies, as the incidence of food allergies among US children nearly doubled to 5.1 percent from 1999 to 2011 and continues to rise (Jackson, Howie, and Akinbami 2013). The responsibility of caretakers like school nurses for keeping children with this potentially fatal condition safe in the presence of triggering foods is growing as the incidence of food allergies rises. Concurrently, the moral implications of technical questions about how to intervene in food allergies using epinephrine auto-injectors are foregrounded and reinforced in food allergy-focused training events like this one held throughout the United States.
This nurse’s concern offers a place to begin unpacking the tangle of prescriptive and implicit responsibilities to protect human lives that are threatened by food allergy. I use the phrase ‘food allergy’ to refer to the biomedically defined condition in which a person’s immune system responds to certain, specific food proteins as though they are dangerous pathogens via a well-characterized, IgE-mediated immune response pathway. The obligation to preserve biological life in moments of allergy-related threats to the body is reinforced by current biomedical orthodoxy, which ties epinephrine use to the promise of more life through food allergy patient education by physicians and pharmaceutical companies. In Joseph Dumit’s (2012) terms, epinephrine, particularly in the form of auto-injector devices, is a ‘drug for life’. Within the food allergy advocacy community, this medical advice meets kinship obligations – especially the obligation of mothers to protect the lives of their children at all costs – to further cement the equivalence between life and epinephrine.
My central argument in this article is that the uses of and discourse surrounding epinephrine auto-injectors in the United States structure relations of care and obligation between individuals with food allergies, their caretakers, their physicians, their communities, and the drug companies that produce the devices. My participants are motivated to use epinephrine in certain ways due to feeling responsible for safeguarding the lives of people with food allergies. Such responsibilities, both enacted and felt, make up the ‘moral’ dimension of epinephrine auto-injectors.
Narrating the moral life of epinephrine
Auto-injectors are understood to be necessary, life-saving biomedical tools by food allergy patients and their physicians, but their moral significance remains largely unexplored, even while it is used to boost sales of the device and to build community among people with, or helping to manage, food allergies in the United States. Previous social scientific accounts of allergy in Europe and North America have examined how patients and researchers interpret the condition as an effect of toxic modernity (Jackson 2007; Raffaetà 2012, 2013), the controversies surrounding the disease category and its effects upon patient identity (Nettleton et al. 2009; Nettleton et al. 2010), and the public imaginary surrounding the peanut allergy ‘epidemic’ (Waggoner 2013). But so far no one has closely examined how this technological object shapes the experience and social connections that result from the disease. Key moments in the life of a person with food allergies revolve around epinephrine: preparing for allergic reactions by learning to use the device, reorienting family and social life around food allergy preparedness, and going online to find support from others who have made it through apparently life-threatening reactions. By focusing on such quotidian activities, my approach responds to Michael Lambek’s (2010, 2) call to study ‘ordinary ethics’: ‘an ethics that is relatively tacit, grounded in agreement rather than rule, in practice rather than knowledge or belief, and happening without calling undue attention to itself’.
Ethics and morality have received a great deal of attention from anthropologists in recent years, with the emphasis placed on documenting and analyzing the varied experience of morality in action rather than on uncovering universal normative parameters for living. For Jarrett Zigon and C. Jason Throop (2014, 3), for example, focusing on the moral dimensions of experienceshifts the working definition of morality from the register of the normative and obligatory, which often draws the attention of moral philosophers and bioethicists, to an ‘attuned concern for the relationality that constitutes our very existence’. A study of moral experience according to them is a study of ‘our experiences of the world and how we might struggle to transform these experiences, to rethink them, to interpret them, to reinhabit them, and to reposition ourselves variously as sufferers or actors on the differing scenes that in part constitute our social existence’ (Zigon and Throop 2014, 8). James Laidlaw (2014), by contrast, seeks a middle ground between ethnographic concern for the practices of embodied life and the work of moral philosophy. He argues that one challenge before anthropologists is to bring together the practice-oriented dimension of morality suggested by Pierre Bourdieu’s (1977) notion of habitus – the idea that what is morally correct is what one learns to do as part of one’s culture- and class-specific, embodied training about how to live – with moral philosophical interest in discovering what counts as virtue and the good.
My own approach to examining the moral life of epinephrine in the context of biomedicine in the United States focuses on how epinephrine figures in caring for people with food allergies. I document the moral code for caring for people with food allergies that circulates among users of auto-injectors. I contend that this code emerges through collective discussions about the lived experience of this condition, and is then used to guide decisions about how to use and talk about these devices. The word ‘moral’ thus indexes two things in this paper: the normative obligations that set forth how one should care for someone with food allergies, and the quality of the experience of carrying out these responsibilities. The responsibility to care situates people with food allergies in particular relation to each other, to caretakers, to medical professionals, to drug companies, and to the public. These relations can change as a drug’s price rises or as the professional norms for patient encounters change, altering what it means to have food allergies or to be a food allergic patient. These two registers of morality – the normative and the practical – coexist in the lives of people living with food allergies.
These practices and discourse surrounding epinephrine auto-injectors are situated in the very specific context of the economics of health care delivery in the United States. In that country, the fact that individuals may have to pay out of pocket for potentially life-saving medications figures prominently in the frameworks patients and caretakers have about what constitutes ‘good’ medical care. Because of the sometimes extraordinary cost of health care today in the United States (a single epinephrine auto-injector now costs upwards of US$300, and patients are advised to carry two injectors with them at all times), debates about the morally correct way to care for a sick person are often enfolded within debates about the appropriate cost of health care. Private health insurance premiums can cost hundreds of dollars per month per person. Lucky Americans with generous coverage provided through an employer have this amount deducted from their pre-tax income, while under the Affordable Care Act freelancers and others whose jobs do not provide insurance and the unemployed are on the hook to pay a similar or higher amount out of their net income or pay tax penalties at year end. Public health care is only available to very poor children and their mothers, people with certain disabilities, and the elderly. For these populations, the trade-off for significantly lower fees is a highly restricted network of providers, which in turn leads to brief appointments at crowded facilities and sometimes waits of weeks or months for needed care.
Cheryl Mattingly’s (2014) recent work on ‘moral laboratories’ – those moments in which different meanings of a ‘good life’ must be negotiated and brought into at least temporary alignment to enable action – is a powerful reminder that contemporary biomedicine is not morally neutral. Medical therapies intervene upon and interact with ordinary, non-expert understandings of the ‘right’ and the ‘good’, as well as with systems of privilege and oppression (Mattingly 2014). Her analysis of experimental moments both within and outside of the clinic among her lower-income, urban, African American participants in the United States shows how mundane encounters between individuals in settings where medical crisis and material need intersect can shape the meaning and practices of care, duty, and hope. Mattingly demonstrates that medical crises that raise urgent questions about how best to care for human bodies are central to some of the most potent and enduring social questions about how best to live among others and make a better life for the next generation of black Americans.
I extend this line of inquiry into the moral content of health care in the United States in a different direction. I consider how biomedical discourse about food allergies and epinephrine, as it is conveyed to and by patients and caretakers of allergic patients, carries with it moral prescriptions about how to be a good caretaker of one’s own body or the body of one’s child. A major difference between Mattingly’s study and mine is the social, economic, and racial context of my participants, who are generally middle- to upper-middle-class white women, most living in nuclear families in rural or suburban areas, content to simply pass on their social status to the next generation. Among them, two moral questions predominate: first, how to manage the biological requirements of life with food allergies; and second, how to live with food allergies – understood as posing a perpetual threat to life – as a social actor embedded in a community. The (frequently at odds) exigencies of biological life and social inclusion must be negotiated, often through discussion about the role of epinephrine in the lives of food allergic individuals.
Arjun Appadurai’s introduction to the 1986 edited volume The Social Life of Things provides some guidance for understanding exactly how a medical commodity can take on new kinds of importance through socially embedded use. Appadurai argues for a shift in thinking about commodities from considering what economic value commodities have qua commodities to understanding how commodities take on economic value through the historical-material fact of being exchanged by people in a society. The Marxian premise that exchange creates value is not enough, however; the methodological challenge for anthropologists, Appadurai (1986, 5) writes, is to ‘follow the things themselves, for their meanings are inscribed in their forms, their uses, their trajectories … [in order to] interpret the human transactions and calculations that enliven things’.
The special problem posed by following medical objects like epinephrine auto-injectors is that their value is always more than economic because their use concerns the well-being of the human body. It is by now a truism among anthropologists that the body is intimately linked to subjective being, social life, and political belonging (Mauss 1973; Scheper-Hughes and Lock 1987; Csordas 1990; Geurts 2002). In diagnosing and curing what ails us physically, high-tech medical interventions thus have the potential to change how an individual experiences the world, understands herself, and relates to others; that is, they shape kinship, non-kin social affiliations, identity groups, and political orientations (Dumit 1997, 2003; Rapp 1999; Healy 2004; Biehl and Moran-Thomas 2009). If bodily experience is, as Csordas (1990, 5) famously argues, ‘the existential ground of culture’, providing the baseline experience of the world which form the foundations for cultural norms and practices and the means by which individuals in a society relate to one another, technologies that enable, prolong, or end human lives are morally potent objects. They have the capacity to make and destroy subjects and social worlds.
This paper narrates the moral life of epinephrine auto-injectors based on nearly two years of multisited ethnographic research (Marcus 1995) with key actors in the food allergy community in the United States. I approached participants based on their involvement in support groups, food allergy education and fundraising nonprofit organizations, conferences and events, and written projects, such as books and blogs about living with food allergies. Early participants put me in touch with other members of the community who were interested in contributing to this research. Participants included parents of food allergic children, food allergic adults, specialty food manufacturing and service entrepreneurs, physicians and nurses, and other professionals who advocate on behalf of those with food allergies. They were overwhelmingly white and socially middle class; most of the parents involved were mothers. The data collected included open-ended, one-on-one interviews by phone and in person, observations through attendance of food allergy conferences and events and shadowing physicians in allergy clinics, and the extensive scientific and autobiographical literatures on allergies suggested by my participants. I also immersed myself in the network of online social networks and blogs where people in the community talk about life with food allergies. This highly ‘polymorphous engagement’ (Gusterson 1997) provided the materials needed to take a comprehensive view of the moral life of epinephrine auto-injectors. The case studies and stories narrated here were chosen because they typify how people use, relate to, and talk about epinephrine auto-injectors and other important aspects of life with food allergies.
Epinephrine in the clinic
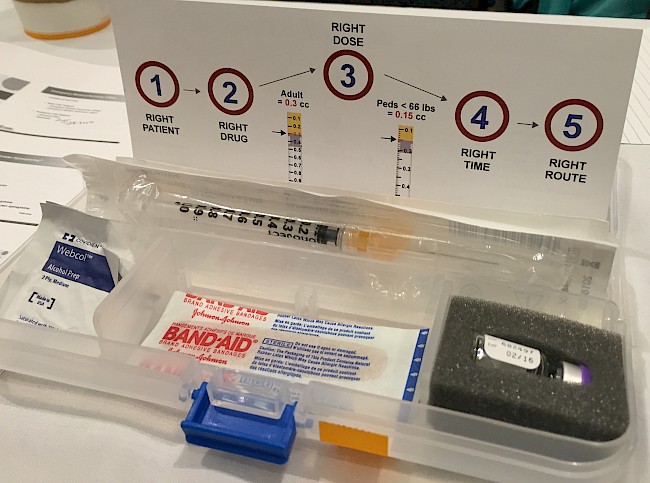
In the global biomedical literature, epinephrine (also known by its Latinate name, adrenaline) is a hormone and neurotransmitter produced by the human body in the adrenal glands. It is said to act on the circulatory system by stimulating the heart muscle and constricting blood vessels (vasoconstriction) and on the nervous system by producing the ‘fight or flight’ response. When administered during the severe, multisystem allergic reaction called ‘anaphylaxis’, epinephrine is understood to stop the blood vessel leakage that causes the symptoms of anaphylaxis, which include hives, redness, and swelling of the airways. Outside of the hospital setting, epinephrine is most often delivered to a patient using an epinephrine auto-injector. These devices are about the size of a marker and consist of a needle, a dose of medication, and a protective plastic case that covers the medication and the needle before and after use. Both global and regional/country-specific medical guidelines throughout the world tell physicians that prompt use of epinephrine is the primary method for reducing the risk of serious injury, such as brain damage, or death resulting from anaphylaxis (Simons et al. 2014). As a result, food allergy patients in the United States are counseled by their physicians to keep two auto-injector devices on their person at all times. The second injector can be used if the first fails, if there is a delay in getting the person to the hospital, or if their reaction is so severe that they continue to react after the first injection.
The typical patient-education episode involving epinephrine auto-injectors in the United States is narrowly focused on pharmaceutical preparedness. It is treated as equivalent to ethical patient care by most biomedical allergists. Once a food allergy is confirmed by medical history, diagnostic tests, or both, the allergist or nurse then demonstrates how to use an epinephrine auto-injector with a ‘trainer’ version of the device. Trainers do not contain medication but have the same mechanism, shape, and size as real auto-injectors. Before and after the rehearsal, the physician advises the patient when to use the auto-injector, the importance of carrying it at all times, and on the need to avoid any foods to which they are allergic. Then the patient leaves, prescription in hand or sent electronically to their pharmacy of choice.
This approach to food allergy preparedness – prescribing a technological object as a quick fix to a complex problem – echoes what science studies scholars have found in studies looking at the technological management of risk in a wide variety of contexts (Jasanoff 1990; Beck 1992; Gusterson 2008; Lakoff 2008; Masco 2008; Adams, Murphy and Clarke 2009; Anderson 2010), including in other areas of biomedicine (Jain 2007; Dumit 2012). As Stefan Timmermans (1999) describes in his history of cardiopulmonary resuscitation (CPR), it takes a great deal of work among medical researchers, educators, and advocates to make a single, heroic medical intervention seem like the obvious solution to a medical crisis. Yet once a quick fix is established in professional practice and the popular imagination as the preferred way to avert death, it is difficult to dislodge. Prescribing epinephrine as the remedy for an acute allergic reaction upon diagnosis of a food allergy does not give people managing food allergies the real-world strategies they typically seek for avoiding the foods they are allergic to in the first place: how to read food labels, what to ask food manufacturers to learn whether allergens have come into contact with food, or how to assertively refuse allergenic foods offered by friends and family members. Particularly conscientious allergists will suggest that the patient consult local support groups or websites but will still offer little practical advice themselves.
The paucity of information most food allergy patients receive about how to live with food allergies feels like an ethical lapse to many on the receiving end of biomedical advice. Indeed, there is push and pull around the use of quick fixes for many disease states in contemporary biomedicine, including within the allergy and immunology specialty. The diabetes and atherosclerosis clinics studied by Annemarie Mol (2002, 2008) in the Netherlands are examples of a kind of health care that does prioritize questions about how best to live with a chronic condition. The clinicians she profiles focus on preventing and slowing the progression of disease by guiding patients toward changing their everyday habits over advocating heroic biomedical interventions, if possible. Social interventions and prevention tactics for asthma, another disease that falls within the professional purview of the allergy and immunology specialty globally, are now hot topics of research and discussion at the medical conferences and physician education events I regularly attended during my research. Yet the normative mode of food allergy advice in particular still advances a heroic logic of medical intervention over a ‘logic of care’ (Mol 2008) that would instead place more emphasis on how to live with food allergy in a way that prevents reactions in the first place.
This is due in large part to the fact that when it comes to treating food allergy, the only robust evidence in the medical literature concerns the administration of epinephrine. For an allergist to go out on a limb to make lifestyle recommendations based solely on patient reports – even if they are the physician’s own patients, and even if she has heard similar stories over and over again – would contravene the evidence-based mood of the modern biomedical clinic (Kaufman 2015) and thereby constitute a kind of ethical breach according to the norms of the specialty. The literature suggests that timely administration of epinephrine during an allergic reaction results in fewer deaths from anaphylactic reactions and that there are no side effects from epinephrine so severe as to prevent its use in any situation of severe allergic reaction (Simons et al. 2013); therefore, timely administration of epinephrine during an allergic reaction is the cornerstone of what most allergists recommend to their patients to manage their disease. From the moment of initiation into the ‘moral career’ of patienthood (Goffman 1961), food allergic people thus encounter epinephrine auto-injectors as the indicator of dutiful self-care for their condition.
Auto-injectors: Lifesavers or commodities?
When I initiated my research in late 2013, there were two epinephrine auto-injectors dominating both the market and the US food allergy community’s imagination: Mylan’s EpiPen (relaunched in its current form by Mylan in 2007) and Sanofi’s Auvi-Q (launched in January 2013 and removed from the market in October 2015). Both work in roughly the same way: the device is removed from a protective case, a piece of plastic is removed from one end that activates the device and makes it possible to expose the needle, and the patient quickly swings the needle end into the large muscles on the outer side of their upper thigh. The device strikes the thigh and clicks, injecting the medication while it is held in place for five to ten seconds. The needle retracts to protect the patient from unnecessary sticks when the device is removed from the body. The devices come in two doses, a 0.3 mg dose intended for children and adults weighing over 66 lbs, and the 0.15 mg version intended for children 15–66 lbs.
Sanofi’s Auvi-Q had a special feature that seemed intended to deepen its connection with the user, which began to form when patients and caretakers learned how to use the device in the clinic. It spoke to users in a prerecorded, slightly mechanical woman’s voice, guiding the user through the process of using the device. It spoke in measured, clearly articulated Standard American English, conveying a sense of calm and competency through careful, unhurried diction. The voice began automatically when the device was removed from its case but paused when it was time for the user to take each action. The voice was there to help, to guide, but not to command. The choice of a female voice suggested a mother, auntie, or nurse who was patiently and willingly inhabiting the female-gendered role of caretaker. The human voice of the Auvi-Q conveyed the message that the user was not alone and would be taken care of in the event of an anaphylactic emergency. The device itself invited the user to entrust it with her life.
The voice feature inspired mixed feelings among my research participants. One allergist with a peanut allergy explained that the product was a much more practical shape to carry in his pocket and had no opinion on the voice. Perhaps the physical design would encourage more people to keep it consistently on their person, he mused when we discussed it. His allergy nurse was a rare dissenter to the general enthusiasm I heard for the product. She expressed discomfort and annoyance with the automated voice, preferring to teach patients to use a different, voiceless device. One parent explained to me that her seven-year-old son was proud to carry his Auvi-Q with him, keeping it in an oversized pocket of his cargo pants. His attachment to the object centers on its technological novelty: for him, it’s not just medicine, it’s his very own gadget that sets him apart from his peers. Since he likes the device, he is never without it, making him a responsible food allergy patient. Many food allergy bloggers (most of whom are mothers of food allergic kids) similarly appreciate a product that guides users through the injection process, especially for small children who may not be able to use the device on their own and may need an untrained adult to help them. The device’s ability to voice instructions widens the circle of people who can be made responsible, even in a moment of crisis, to care for someone with serious food allergies. This, in turn, is understood to widen the social horizons for food allergic children in particular, making activities like sports and summer camps safer and therefore more accessible. The voice feature gave the Auvi-Q an edge over the EpiPen for some. For others, the longer, trustworthy history of the EpiPen brand, personal and community familiarity with it, or inertia around changing their prescription when the newer device was released precluded adoption of the Auvi-Q.
In addition to being familiar fixtures of everyday life with food allergies, epinephrine auto-injectors are big business for the pharmaceutical companies who make them. The price consumers pay for epinephrine auto-injectors, as for all prescription pharmaceuticals and medical devices in the United States, results from a complicated calculation. With private insurance, it is common to be charged a US$10–20 ‘co-pay’ for every doctor’s visit, service, or filled prescription that is covered, with potentially many charges stacking from a single visit or prescription. Many people only access such benefits after paying a deductible of the first several thousand dollars of care each calendar year, and this is especially the case for those employed by smaller companies or purchasing insurance on their own. Many prescriptions and services are not covered or carry higher fees, especially brand-name and specialty products, two categories which include epinephrine auto-injectors. In this context, one pediatrician has estimated that treating food allergies alone costs more than US$25 billion per year – more than US$4,000 per food allergy family – in special food, emergency medical costs, and doctor’s visit and drug co-pays (Gupta et al. 2013). These out-of-pocket costs translate into profits for insurance companies (via co-pays and deductibles), testing companies, healthcare providers, and drug and device makers (via direct payments for non-covered goods and services).
When looking at the United States healthcare system as a whole, the presumed care orientation of biomedicine is increasingly subsumed to the market logic of health care economics, where what is used in medical practice is what both works against expensive, strategically designed, placebo-controlled studies (Dumit 2012) and what can be justified by financial modeling (Kaufman 2015). Food allergy is no exception to this story. In this environment, objects that patients see as lifesaving resources can appear from other perspectives to be merely the means by which to generate capital – that is, they are fetishized as commodities (Marx 1976). In the second half of 2015, two news reports shook the food allergy community by revealing the commodity status of epinephrine auto-injectors. This media coverage forced reflection on the fact that epinephrine auto-injectors have different kinds of value to different actors: they are both a morally potent, life-saving, but expensive companion for people with food allergies and a profit-generating commodity for the companies who produce them.
The first of these two reports was an investigative piece released by the news outlet Bloomberg Business on 23 September 2015 (Koons and Langreth 2015). Their research confirmed what I had been hearing from food allergy advocates for months: the price of EpiPens had increased significantly. It had risen from US$57 per device in 2007 when Mylan acquired the device to a typical price of US$415 for a two-pack in 2015. The rise in prices, according to the Bloomberg report, contributed to a jump in revenue generated by the product, from US$200 million in 2007 to over US$1 billion with a 55 percent profit margin in 2015. It now made up 40 percent of Mylan’s profits. Its ‘brand equity’ – the quantified value of product loyalty it inspires in customers – made it impervious to competition from a less expensive generic version, according to CEO Heather Bresch. In the first half of 2015, the device had an 85 percent claim on the market. For the company, the EpiPen epinephrine auto-injector is a hot commodity, one whose profitability is ensured by its image as a trustworthy, life-saving device.
Mylan and Sanofi made frequent use of co-pay discount cards to allay financial anxieties among users. This marketing-tool-cum-social-support-program is used extensively for branded pharmaceuticals. Co-pay discount cards reduce the amount paid by patients with insurance coverage by a specified amount (often by US$100 for auto-injectors). This helps customers with good insurance coverage that leaves them with reasonable co-pays for prescriptions, but does little for those with high deductibles or poor prescription coverage, and it does nothing for the uninsured or for patients who happen to miss the news about co-pay promotions. While these programs were originally rolled out as temporary promotions by both companies on the heels of the Auvi-Q’s release in early 2013, the expiration dates on these programs have been extended again and again, with new cards being distributed at conferences, in doctors’ offices, and even directly to patients online. High asking prices for the devices now seem to exist in perpetual balance with generous co-pay subsidies from the companies themselves. Mylan and Sanofi could thus appear to care for the economic situation of their customers, yet still reap significant profits from these devices quarter after quarter from the remaining fees and from consumers left out of the loop. They are far from alone among pharmaceutical companies; programs oriented toward so-called corporate citizenship or corporate social responsibility that claim to enrich the lives of the communities in which they operate and broaden access geographically and socioeconomically form a growing corporate strategy in the sector (Ecks 2008; Dietrich 2013).
A second news item added to the worries of people with food allergies when, on 28 October, Sanofi announced an enormous recall of all Auvi-Q devices on the market (Sanofi 2015). The recall underscored for many in the food allergy community that these devices are potentially fallible, and access to them is subject to the whims and exigencies of corporate strategy. One blog post on the food allergy advocacy website, Oh Mah Deehness!, run by an attorney active in food allergy advocacy in her state, is representative of the reactions among food allergy bloggers. In a blog post titled, ‘All Auvi-Q Epinephrine Autoinjectors Recalled’, she wrote, ‘You could view the recall as being in an abundance of caution and therefore showing how sensitive Sanofi is being to consumers or you can wonder about the timing and the lack of actual clear instructions for how to handle the issue’ (Woodrum 2015). She reports that the recall came less than a week after she received an email from the company encouraging her to purchase more of their devices before the end of a promotional pricing program, piquing her suspicion about their motives.
The high price of auto-injectors at the time of the recall made upcoming decisions about how best to care for her daughter both moral and economic decisions:
The costs of replacing medication, between new doctor’s appointments for prescriptions, potentially missing work or school to sort matters out, and more are factors that Sanofi can’t control. I have preferred the Auvi-Q in form and function since it was released but will be sending my daughter to school tomorrow with a set of EpiPens. The sheer expense of a mid-year replacement (ie, replacing the EpiPen with an Auvi-Q if new batches ship) make reverting back to the Auvi-Q another year away for us. (Woodrum 2015)
How much money can be spent on following medical guidance to make sure her daughter carries a set of two auto-injectors at all times, even for a comfortably middle-class lawyer?
This tension between financial value and the practical, life-saving usefulness of epinephrine auto-injectors poses serious problems for people living with food allergies. In particular, how can patients or caretakers comply with medical advice to have two auto-injectors with the allergic person at all times (which often means buying duplicate pairs for school and home or home and work) and still have the personal financial resources to live the good life that maintaining their health is intended to ensure? What are the financial stakes of using an auto-injector when a reaction is suspected but not confirmed – should one risk wasting hundreds of dollars or more to give an injection if it is a false alarm? What of the hundreds to tens of thousands of dollars (depending on local rates and insurance coverage) the doctor-recommended ambulance ride and stay in the emergency room will cost? Between learning how to use these life-saving devices, learning when they ought to use them, and making financially conscious decisions about how and when to use them, patients find themselves in new configurations with physicians, nurses, caretakers, and multinational pharmaceutical companies. This social terrain is shot through with responsibilities and expectations about the best way to care for an allergic body, and it revolves around epinephrine auto-injectors, lively objects with multiple kinds of value and meaning.
Death talk: Epinephrine as savior in the face of human fallibility
The moral code of the food allergy community in the United States is another key aspect of the moral life of epinephrine. This code echoes biomedical wisdom: epinephrine is a life-saving medication, and epinephrine auto-injectors ought to be used whenever anaphylaxis occurs, even if it does not initially appear to be severe. ‘Death talk’ is one of the main vehicles for communicating this doctrine; it is a hallmark of food allergy activist texts and one of the threads that holds people together within this patient world. I learned this term from a participant with whom I became close during my research. A trained social worker and food allergy therapist, her life revolved around food allergies, yet she tried to resist the fear many felt while living with the condition. While food allergies can sometimes lead to the serious, multisystem allergic reaction called anaphylaxis, and while anaphylaxis can sometimes lead to death, death talk makes food allergy, anaphylaxis, and death seem identical in all cases. The dramatization of the danger of food allergies and the power of epinephrine serve an explicitly normative role: to teach the reader about the ‘proper’ role of epinephrine in food allergic life.
Death talk is abundant on food allergy blogs. One post that exemplifies the genre appeared on the Allergy Eats! blog during the summer of 2014. Titled ‘Our First Experience with the Epi – All the Details and 16 Lessons to Take Away’, the piece tells the story of what happened when the writer’s teenaged son ate a cookie containing hidden nuts at a party (Antico 2014). At first, the son and parents don’t believe the reaction is severe and treat it with Benadryl. Eventually the son senses that something may be seriously wrong and the parents call 911 for an ambulance to take him to the hospital. The reaction recurs hours later, to the surprise of both the family and the emergency room staff.
The ‘16 lessons’ that punctuate the post are written in bold, italicized text. Epinephrine turns up in eight of them. Lesson 2 states, ‘Teach your teens to carry their autoinjectors. Help them find a way to avoid any embarrassment they may feel. Have them read this story if necessary’. Lesson 7 instructs the reader, ‘When you decide to use the epi, be strong in your conviction – you’re doing the right thing. Don’t hesitate!’ This is echoed by Lesson 13: ‘Did I mention – Don’t Fear Using the Epi! Fear NOT using it!’ The writer ultimately credits the food allergy community with equipping him with knowledge about the importance of being prepared to use epinephrine auto-injectors.
Another unsettling example of death talk is a periodically updated blog post on the online support website No Nuts Moms Group (Rutter 2014). Titled ‘Remembering Those We Have Lost to Food Allergies’, the post consists of a list of names in bold, red text, followed by age, cause of death, and a link to a relevant online news article, arranged by the year of death. A typical entry reads, ‘Michael Saffioti, 22, died from dairy allergy after eating oatmeal containing dairy’. Another, from 2008, features more theatrical language: ‘Daniel Sargent, 30, collapsed after taking a bite of a chocolate chip cookie’. The news stories they link to describe the events leading up to the person’s death and some basic facts about their life, and explain that food allergy is apparently on the rise in the United States. In the comments section (which had forty-one posts in November 2015), some readers express their gratitude for the list, while others report that the list drove home for them the seriousness of food allergies. This list of worst-case outcomes provides evidence of what happens when patients do not follow recommendations for epinephrine use. Its message is that there is a straight line from a food allergy diagnosis, to allergen exposure leading to anaphylaxis, to death.
The pedagogical aims of these two popular posts are important to the writers. Among people with food allergies and parents of allergic children, there is a widely shared (and sometimes confirmed) fear that food allergies are not taken seriously by people without the condition. ‘Educating’ the public about food allergy via morality tales about epinephrine is seen as a way to teach people unaffected by allergies how serious the condition can be. By making audiences ‘more aware’ of the dangers of food allergy and of the necessity to use epinephrine to reverse reactions, food allergy writers are also seeking to make them more responsible for those around them who may have food allergies. Longer term, and like other patient advocacy movements, raising awareness is understood to bring greater attention from researchers and greater representation in political debates about the distribution of limited health care resources (Rabeharisoa and Callon 2002; Dumit 2006; Landzelius 2006; Rabeharisoa 2006; Panofsky 2011).
A second function of these stories is to bring people living with food allergies – especially mothers of allergic children – together through narrations of their shared food allergy experiences. The closing appeal of the Allergy Eats! blog post reveals this function:
I have learned so much over the years reading and hearing about everyone else’s experiences – the right decisions and the wrong decisions. We are all in this together! And if I hadn’t learned from you that giving an epi too late can be fatal, that epinephrine can’t harm your child, that the relief of an epi is virtually instantaneous, that getting an anaphylactic sufferer off his feet is important, that calling 911 after administering an epi is ‘mandatory,’ etc., well… thank you for teaching me! (Antico 2014)
Sharing testimonials about epinephrine use – including failures and near-misses – via email listservs and social media posts provides a way for people managing food allergies to build and maintain a network of support and advocacy beyond their families and local communities.
Death talk stories are also passed from person to person in private moments. In these moments the anxiety concerning how best to adhere to the normative expectations for epinephrine auto-injector use becomes most apparent. During one phone interview, a nursing professional whose son has severe food allergies, who I will call Shelly, told me a detailed story about her son’s first anaphylactic reaction as a baby. Rather than administering epinephrine and taking him to the hospital, she gave him an oral antihistamine – an old mode of treatment that is no longer recommended for anaphylaxis, though it works for milder, environmental allergies – and monitored him closely for over a day. Already familiar with the signs and symptoms of medical crises from her training, she managed the superficial symptoms, like facial swelling, but began to worry when too many hours passed without her son producing urine – a sign of kidney failure. At this point Shelly began to doubt and regret her decision not to give epinephrine, which she made because she wanted to avoid the time and cost of a trip to the emergency room and because she had confidence in her ability to handle a medical emergency if it occurred. His condition soon reversed, but she described it to me as a close call. She would not advise anyone else to make the same decisions she had, even though, in practice, things turned out well for her son.
The morality of much public food allergy death talk obscures many of the complexities about using auto-injectors, implying that most choices, accidents, and resistance to following the dictates of biomedical authority to the letter are nothing more or less than negligence. It additionally covers up the commodity status of epinephrine auto-injectors to the companies that make them, and by extension makes it difficult for users, prescribers, and patient advocacy organizations to critique the financial burden their rising costs place on families and patients. Stories like Shelly’s illustrate that the morality of food allergy care does not capture the complexity of making real-life decisions about how to responsibly respond to an allergic crisis. Shelly’s expertise in managing medical crises created a loophole that allowed her to avoid using an auto-injector and going to the hospital. Since everything turned out all right for her son, her choice appeared responsible in hindsight.
Morality and biomedicine in the United States
Epinephrine auto-injectors structure relations of care between those with food allergies, those who care for them, and those who play supporting roles in the drama of living with food allergies in the United States. Analyzing their role in the social worlds of American food allergy sufferers makes it clear that they are more than just high-tech, quick-fix tools. They have value that is more complex – both morally and financially – and more socially fraught than the price printed on a health insurance plan’s price list. I call the activities and discussions with and around these devices the ‘moral life’ of epinephrine to draw attention to the epinephrine auto-injector’s ability to configure and sustain social relations: they are lively objects, not dead commodities.
The consequences of these social configurations are both good and bad. Death talk, for example, makes many of my participants fearful and see their possibilities for a ‘normal’ life as very limited. Patients are also undoubtedly being exploited by the drug-pricing practices of auto-injector manufacturers, and companies’ messages that claim they are on the side of patients only add insult to injury. On the other hand, the financial resources and political access of multinational drug companies to United States policymakers provide openings for advocacy groups, charismatic physicians, and patients to share their experiences with legislators to push for more universal health care coverage and public health measures to limit the impact of a food allergy diagnosis, including the provision of auto-injectors in public spaces for anyone to use. An ethic of collective and accessible care thus arises from, and coexists with, the heroic, individualized ethic of current biomedical best practice.
The moral life of epinephrine is not reducible to a clear message about what constitutes proper care for people with food allergies, what they should do to manage their disease, or with whom they should join forces to do so. Following epinephrine devices reveals that managing food allergies involves negotiations about financial value, biomedical authority, doctor-patient relationships, and relations with loved ones and community members. It also means coming to terms with the device itself: learning how to use it, getting accustomed to its presence and voice, and making decisions about how and when to use it in a food allergy crisis. These questions are, in part, moral questions, that is, questions about how to make and maintain social worlds when one’s life is mediated by expectations about the responsibility of patients and caregivers. Analyzing this medical technology offers a potent space to think through questions about the everyday moral stakes of living with an illness in a biomedical society.
About the author
Danya Glabau earned her PhD from the Department of Science and Technology Studies at Cornell University. Her dissertation, ‘The Moral World of Food Allergies in the United States’, blends STS and medical anthropology approaches to understand the food allergy advocacy and research communities in the United States. Her book project combines her dissertation research on food allergy activism with a deeper examination of how the logics of global finance shape drug development and the definition of ‘good’ medical care for food allergies. She is currently core faculty at the Brooklyn Institute for Social Research.
References
Adams, Vincanne, Michelle Murphy, and Adele E. Clarke. 2009. ‘Anticipation: Technoscience, Life, Affect, Temporality’. Subjectivity 28, no. 1: 246–65. https://doi.org/10.1057/sub.2009.18.
Anderson, Ben. 2010. ‘Preemption, Precaution, Preparedness: Anticipatory Action and Future Geographies’. Progress in Human Geography 34, no. 6: 777–98. https://doi.org/10.1177/0309132510362600.
Antico, Paul. 2014. ‘Our First Experience With the Epi – All the Details and 16 Lessons to Take Away’. The Allergy Eats! Blog, 21 May. Accessed 24 November 2016. http://www.allergyeats.com/blog/index.php/our-first-experience-with-the-epi-all-the-details-and-16-lessons-to-take-away/.
Appadurai, Arjun. 1986. ‘Introduction: Commodities and the Politics of Value’. In The Social Life of Things: Commodities in Cultural Perspective, edited by Arjun Appadurai, 3–63. Cambridge: Cambridge University Press.
Beck, Ulrich. 1992. Risk Society: Towards a New Modernity. Newbury Park, CA: SAGE Publications.
Biehl, João, and Amy Moran-Thomas. 2009. ‘Symptom: Subjectivities, Social Ills, Technologies’. Annual Review of Anthropology 38, no. 1: 267–88. https://doi.org/10.1146/annurev-anthro-091908-164420.
Bourdieu, Pierre. 1977. Outline of a Theory of Practice. New York: Cambridge University Press.
Csordas, Thomas. 1990. ‘Embodiment as a Paradigm for Anthropology’. Ethos 18, no. 1: 5–47. https://doi.org/10.1525/eth.1990.18.1.02a00010.
Dietrich, Alexa S. 2013. The Drug Company Next Door: Pollution, Jobs, and Community Health in Puerto Rico. New York: New York University Press.
Dumit, Joseph. 1997. ‘A Digital Image of the Category of the Person: PET Scanning and Objective Self-Fashioning’. In Cyborgs & Citadels: Anthropological Interventions in Emerging Sciences and Technologies, edited by Gary Lee Downey and Joseph Dumit, 83–102. Santa Fe, NM: School of American Research Press.
Dumit, Joseph. 2003. ‘Is It Me or My Brain ? Depression and Neuroscientific Facts’. Journal of Medical Humanities 24, no. 1/2: 35–47. https://doi.org/10.1023/A:1021353631347.
Dumit, Joseph. 2006. ‘Illnesses You Have to Fight to Get: Facts as Forces in Uncertain, Emergent Illnesses’. Social Science & Medicine 62, no. 3: 577–90. https://doi.org/10.1016/j.socscimed.2005.06.018.
Dumit, Joseph. 2012. Drugs for Life: How Pharmaceutical Companies Define Our Health. Durham, NC: Duke University Press.
Ecks, Stefan. 2008. ‘Global Pharmaceutical Markets and Corporate Citizenship: The Case of Novartis’ Anti-Cancer Drug Glivec’. BioSocieties 3, no. 2: 165–81. https://doi.org/10.1017/s1745855208006091.
Geurts, Kathryn Linn. 2002. Culture and the Senses: Bodily Ways of Knowing in an African Community. Berkeley: University of California Press.
Goffman, Erving. 1961. Asylums: Essays on the Social Situation of Mental Patients and Other Inmates. New York: Anchor Books.
Gupta, Ruchi, David Holdford, Lucy Bilaver, Ashley Dyer, Jane L. Holl, and David Meltzer. 2013. ‘The Economic Impact of Childhood Food Allergy in the United States’. JAMA Pediatrics 167, no. 11: 1026–31. https://doi.org/10.1001/jamapediatrics.2013.2376.
Gusterson, Hugh. 1997. ‘Studying Up Revisited’. PoLAR 20: 114–19. https://doi.org/10.1525/pol.1997.20.1.114.
Gusterson, Hugh. 2008. ‘Nuclear Futures: Anticipatory Knowledge, Expert Judgment, and the Lack That Cannot Be Filled’. Science and Public Policy 35, no. 8: 551–60. https://doi.org/10.3152/030234208X370639.
Healy, David. 2004. ‘Shaping the Intimate: Influences on the Experience of Everyday Nerves’. Social Studies of Science 34, no. 2: 219–45. https://doi.org/10.1177/0306312704042620.
Jackson, Mark. 2007. Allergy: The History of a Modern Malady. London: Reaktion Books Ltd.
Jackson, Kristen D., LaJeana D. Howie, and Lara J. Akinbami. 2013. NCHS Data Brief: Trends in Allergic Conditions Among Children: United States, 1997–2011. NCHS Data Brief, May 2013. Hyattsville, MD: National Center for Health Statistics.
Jain, Sarah Lochlann. 2007. ‘Living in Prognosis: Toward an Elegiac Politics’. Representations 98, Spring: 77–92. https://doi.org/10.1525/rep.2007.98.1.77.
Jasanoff, Sheila. 1990. ‘American Exceptionalism and the Political Acknowledgment of Risk’. Daedalus 119, no. 4: 61–81.
Kaufman, Sharon. 2015. Ordinary Medicine: Extraordinary Treatments, Longer Lives, and Where to Draw the Line. Durham, NC: Duke University Press.
Koons, Cynthia and Roberg Langreth. 2015. ‘How Marketing Turned the EpiPen into a Billion-dollar Business’. Bloomberg Business, 23 September. Accessed 24 November 2016. http://www.bloomberg.com/news/articles/2015-09-23/how-marketing-turned-the-epipen-into-a-billion-dollar-business.
Laidlaw, James. 2014. The Subject of Virtue: An Anthropology of Ethics and Freedom. New York: Cambridge University Press.
Lakoff, Andrew. 2008. ‘The Generic Biothreat, Or, How We Became Unprepared’. Cultural Anthropology 23, no. 3: 399–428. https://doi.org/10.1111/j.1548-1360.2008.00013.x.
Lambek, Michael. 2010. ‘Introduction’. In Ordinary Ethics: Anthropology, Language, and Action, edited by Michael Lambek, 1–36. New York: Fordham Unviersity Press.
Landzelius, Kyra. 2006. ‘The Incubation of a Social Movement? Preterm Babies, Parent Activists, and Neonatal Productions in the US Context’. Social Science & Medicine 62, no. 3: 668–82. https://doi.org/10.1016/j.socscimed.2005.06.024.
Marcus, George E. 1995. ‘Ethnography in/of the World System: The Emergence of Multi-Sited Ethnography’. Annual Review of Anthropology 24: 95–117.
Marx, Karl. 1976. Capital. Volume I. Translated by Ben Fowkes. New York: Penguin Books.
Masco, Joseph. 2008. ‘“Survival Is Your Business”: Engineering Ruins and Affect in Nuclear America’. Cultural Anthropology 23, no. 2: 361–98. https://doi.org/10.1111/j.1548-1360.2008.00012.x.
Mauss, Marcel. 1973. ‘Techniques of the Body’. Economy and Society 2, no. 1: 70–88. https://doi.org/10.1080/03085147300000003.
Mattingly, Cheryl. 2014. Moral Laboratories: Family Peril and the Struggle for a Good Life. Oakland, CA: University of California Press.
Nettleton, Sarah, Brian Woods, Roger Burrows, and Anne Kerr. 2009. ‘Food Allergy and Food Intolerance: Towards a Sociological Agenda’. Health (London) 13, no. 6: 647–64. https://doi.org/10.1177/1363459308341433.
Nettleton, Sarah, Brian Woods, Roger Burrows, and Anne Kerr. 2010. ‘Experiencing Food Allergy and Food Intolerance: An Analysis of Lay Accounts’. Sociology 44, no. 2: 289–305. https://doi.org/10.1177/0038038509357208.
Panofsky, Aaron. 2011. ‘Generating Sociability to Drive Science: Patient Advocacy Organizations and Genetics Research’. Social Studies of Science 41, no. 1: 31–57. https://doi.org/10.1177/0306312710385852.
Rabeharisoa, Vololona. 2006. ‘From Representation to Mediation: The Shaping of Collective Mobilization on Muscular Dystrophy in France’. Social Science & Medicine 62, no. 3: 564–76. https://doi.org/10.1016/j.socscimed.2005.06.036.
Rabeharisoa, Vololona, and Michel Callon. 2002. ‘The Involvement of Patients’ Associations in Research’. International Social Science Journal 54, no. 171: 57–63. https://doi.org/10.1111/1468-2451.00359.
Raffaetà, Roberta. 2012. ‘Conflicting Sensory Relationships. Encounters with Allergic People’. Anthropology & Medicine 19, no. 3: 339–50. https://doi.org/10.1080/13648470.2012.692359.
Raffaetà, Roberta. 2013. ‘Allergy Narratives in Italy: “Naturalness” in the Social Construction of Medical Pluralism’. Medical Anthropology 32, no. 2: 126–44. https://doi.org/10.1080/01459740.2012.732632.
Rapp, Rayna. 1999. Testing the Woman, Testing the Fetus: The Social Impact of Amniocentesis in America. New York: Routledge.
Rutter, Lisa. 2015. ‘Remembering Those We Have Lost To Food Allergies’. Not Nuts Moms Group Blog, 21 September. Accessed 24 November 2016. http://nonutsmomsgroup.weebly.com/blog/remembering-those-we-have-lost-to-food-allergies.
Sanofi. 2015. ‘Sanofi US Issues Voluntary Nationwide Recall of Auvi-Q Due to Potential Inaccurate Dosage Delivery’. Accessed 24 November 2016. http://www.news.sanofi.us/2015-10-28-Sanofi-US-Issues-Voluntary-Nationwide-Recall-of-Auvi-Q-Due-to-Potential-Inaccurate-Dosage-Delivery.
Scheper-Hughes, Nancy, and Margaret Lock. 1987. ‘The Mindful Body: A Prolegomenon to Future Work in Medical Anthropology’. Medical Anthropology Quarterly 1, no. 1: 6–41. https://doi.org/10.1525/maq.1987.1.1.02a00020.
Sicherer, Scott H., and Hugh A. Sampson. 2010. ‘Food Allergy’. The Journal of Allergy and Clinical Immunology 125, no. 2, Suppl. 2: S116–25. https://doi.org/10.1016/j.jaci.2009.08.028.
Simons, F. Estelle R., Ledit R. F. Ardusso, Vesselin Dimov, Motohiro Ebisawa, Yehia M. El-Gamal, Richard F. Lockey, Mario Sanchez-Borges, et al. 2013. ‘World Allergy Organization Anaphylaxis Guidelines: 2013 Update of the Evidence Base’. International Archives of Allergy and Immunology 162: 193–204. http://dx.doi.org/10.1159/000354543.
Simons, F. Estelle R., Ledit R. F. Ardusso, M. Beatrice Bilò, Victoria Cardona, Motohiro Ebisawa, Yehia M. El-Gamal, Phil Lieberman, et al. 2014. ‘International Consensus on (ICON) Anaphylaxis’. World Allergy Organization Journal 7, no. 1: 9. http://dx.doi.org/10.1186/1939-4551-7-9.
Timmermans, Stefan. 1999. Sudden Death and the Myth of CPR. Philadelphia: Temple University Press.
Waggoner, Miranda R. 2013. ‘Parsing the Peanut Panic: The Social Life of a Contested Food Allergy Epidemic’. Social Science & Medicine 90: 49–55. https://doi.org/10.1016/j.socscimed.2013.04.031.
Woodrum, Homa. 2015. ‘All Auvi-Q Epinephrine Autoinjectors Recalled’. Oh Mah Deehness!, 28 October. Accessed 24 November 2016. https://ohmahdeehness.wordpress.com/2015/10/28/102815-all-auvi-q-epinephrine-autoinjectors-recalled/.
Zigon, Jarrett, and C. Jason Throop. 2014. ‘Moral Experience: Introduction’. Ethos 42, no. 1: 1–15. https://doi.org/10.1111/etho.12035.