Milk, meaning, and morality
Following the trajectory of donated breast milk from donor to baby
—
Abstract
‘Part food, part medicine, part human tissue’ is how Dr Muller [note 1] described what he called ‘breast milk’,[note 2] in our first meeting in a second-tier maternity hospital in Cape Town, South Africa in 2014. South Africa’s stratified public health care system consists of primary health care facilities (clinics and antenatal units), a second tier of hospitals with some specialist services and third tier (academic) hospitals that offer comprehensive services and teaching functions. The hospital in which we conducted part of the research combines second- and third-tier functions. As a metropolitan state hospital it is considered well-resourced, but not by comparison to the private sector. We were there to establish a project that would trace milk from its donors to recipient infants in a neonatal intensive care unit. Muller, a specialist paediatrician, worked in the ICU and was a board member of a milk bank (henceforth, the milk bank) that distributed human milk. It is one of forty-four in South Africa, most of which are housed in state hospitals. The Milk Bank in which we conducted research is operated by a nongovernmental organisation in Cape Town, supplying about forty babies at a time across six different hospitals in Cape Town (mainly large public hospitals). Sitting around a table at the milk bank, Muller explained that between eight hundred to one thousand babies were birthed at the hospital every month and, of these, about one hundred and fifty were admitted to ‘the nursery’, which included intensive care, high care, special care, and ‘kangaroo care’ facilities,[note 3] for periods ranging from a few days to several months. Most of these infants were born into Cape Town’s lower socioeconomic strata. Approximately thirteen per cent were exposed to HIV, of whom one or two would later seroconvert, and one or two per month were born HIV positive. At any given time, between two and ten of the nursery infants would be receiving donated human milk on prescription, although at peak times that number might increase to twenty.
Muller was animated and serious as he described the milk bank’s strategies for securing and distributing milk. Milk banks in general face shortages of donated milk and cannot completely meet hospitals’ demands. Strict criteria determine which infants receive donated milk, and milk banks need constantly to recruit donors. The milk bank that is the focus of this research was run under medical supervision by a small group of women, some employed, some volunteer, and was one of the hubs for milk distribution in the region. Four of the five women were trained as nurses, lactation consultants, or dieticians. Their enthusiasm for their work and the virtues of human milk was inspiring. Equally so was the dedication of Dr Wilson, a neonatologist in charge of the ICU, who showed us the tiny infants who would receive donated milk. The scene was a far cry from the domestic settings in which the donors who participated in this research expressed and stored milk prior to sending it off to the Milk Bank and hospital system to ‘save’ needy babies.
The research followed donated milk’s trajectory from donating mother through its emergence as an alienated product, its incorporation in the clinic setting, and its distribution and reception. By following its path, different actors, institutions, and practices are rendered visible. Our work demonstrates the shifts in milk’s meanings, and possibly the temporary transformation of its status, as the unique mother-child dyad in which it is produced becomes open to others, as an intimate substance becomes generalisable. Our empirical work demonstrates how human milk is conceptualised at different points in this network, mapping the modes of intimacy and care that it frames and enables. The milk, offered by donors as a gift, is traced through the extended route that takes it from breast to mouth in a political economic context that shapes who donates (mostly middle-class women outside of hospitals) and who receives (mostly sick or needy newborns of working-class women).
Known as ‘liquid gold’ (see Carroll 2014) for its capacity to respond to the specific demands of a hungry, growing infant, human milk’s status as a complete food with immune-boosting capacity is intensified by the psychological and cognitive effects of bonding and care during feeding (see Else-Quest, Shibley Hyde, and Clark 2003; Jansen, De Weerth, and Riksen-Walraven 2008; Marshall, Godfrey, and Renfrew 2007; Smith and Ellwood 2010). When this intimate substance is distanced from the particular mother-child dyad its multiple referents are foregrounded differently as it enters a chain of distribution, but its inherent ambiguities are never fully transformed. The chain of distribution is carefully managed; milk must be alienated but not commodified as it may not be bought or sold in South Africa.[note 4] In this context consubstantiation is both acknowledged (human milk is preferred even when the baby is not biogenetically related) and denied (in that the donor and recipient baby do not meet). Alongside this arise concerns about contamination, infection, and issues of repulsion. This article explores how these issues materialise and are dealt with through the technical processes and moral discourses that accompany milk donation and receipt. We argue that together these shift the intrinsic ambiguity of milk, consolidating its status differently as food, bodily fluid, and medicine at different points in its trajectory from donor to recipient.
There is currently a strong international movement promoting breastfeeding worldwide. This position is reflected in South African state policy (Department of Health 2013) and official declarations of the World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF). After a decade of promoting the use of formula milk rather than human milk, a decision made in light of high infant mortality rates (forty-one deaths per 1,000 births in 2015 according to World Bank (2016) data) and the risks of vertical (mother-to-child) HIV transmission, the South African government recommitted to facilitating breastfeeding in the Tshwane declaration of August 2011 (National Breastfeeding Consultative Meeting 2011). This was a direct result of the revised guidelines on breastfeeding published by the WHO in 2010 (Doherty et al. 2011), which were informed by South African research (see Coovadia et al. 2007; Coutsoudis et al. 2001; Thairu et al. 2005). In South Africa, only 61 percent of newborns are fed human milk (UNICEF 2012), and only 8 percent of mothers are reported as breastfeeding exclusively until six months, as recommended by the state and the WHO (National Breastfeeding Consultative Meeting 2011; Doherty et al. 2011). State and hospital support for breastfeeding are crucial: even in a context of privilege, social support, and economic wealth breastfeeding is no easy task or sure success (see Schmied and Lupton 2001; Waltz 2013, 2014).
South Africa’s Gini coefficient (a measure of inequality) is 63.3, the highest in the world. Access to health care is limited for a significant proportion of the population. However, the birthing sector is highly medicalised: more than 80 percent of babies are born in a medical facility, although quality of care varies considerably and maternal and infant morbidity and mortality rates do not yet meet the Millennium Development Goals. A large divide exists between public and private health care systems. Nevertheless, although the public sector faces a lack of resources and overburdened facilities, it is sometimes at the forefront of new developments, including Baby Friendly Hospitals (an initiative of the WHO and UNICEF based on the Innocenti Declaration of 1990, see UNICEF 2005) and Kangaroo Mother Care. It is against this backdrop that the trajectory of donated breast milk enters the entanglement of scientific recommendations, economic calculations, and moral imperatives.
Located in a middle-class suburb in Cape Town, the public hospital that hosted our research has a century-long history of providing maternity services. Its obstetric and neonatal services are available to women who have been referred from primary healthcare facilities on the basis of complications in pregnancy. The hospital has a proud reputation for its maternity functions and ‘baby-friendly’ status. Its patients are mostly working class or very poor. Fifteen to 18 percent of babies delivered at the hospital are born prematurely. Premature babies, especially those weighing less than 1.5 kilograms, who are fed formula milk (even when it is especially designed for premature babies) are highly susceptible to necrotising entrocholitis (Neu and Walker 2011). Many mothers of babies at the hospital may not settle into breastfeeding in the early weeks if at all, because they do not have regular access to the hospital, or are malnourished, too sick, or drug or alcohol dependent. Donated human milk offers a critical intervention into securing life; as the slogan goes, ‘breast is best’. For these babies, receiving donated milk is not merely about often-cited advantages such as higher IQ, stronger physical health, and better bonding (Blum 1999; Hinde and German 2012) but can be a matter of life and death.
Milk is produced in the context of a particular mother and a particular child, and is shaped by the environment; there is a direct relation between outside and inside. Infants receive ‘personalised’ milk from their mother (Hinde and German 2012, 92). What a mother eats and drinks, how much she sleeps and exercises, what coping networks exist for her, and how she feels all impact on the constitution of the milk she produces. Affective and political economies are materialised in this intimate substance. Additionally, an individual mother’s milk changes from feed to feed to address the particular needs of her baby (Garbes 2015). Through the process of donation, milk leaves the closed circuit between mouth and breast and enters a circuit that makes it generalisable. Generalisability is produced through discourses, technologies, and practices that materialise around the object and change it, both literally and figuratively. We have identified four nodes at which its status is reworked, each of which is effected through discursive and technological interventions and is accompanied by a different moral orientation, the overarching theme of which is ‘saving life’. We show that while never fully settled, these interventions temporarily foreground one or two of milk’s multiple meanings to enable it to ‘perform’ efficaciously in the different contexts of its use.
Our ethnographic research focused on three groups of participants: donating mothers, staff from the milk bank and hospital, and recipient mothers. Ten ‘private’ donors – breastfeeding mothers whose babies were not patients at the hospital – were interviewed. Eight were white. In Cape Town, the majority of private donors were (upper-) middle-class, highly educated, in their thirties, and living in nuclear families (see Waltz 2013). This parallels research findings elsewhere (Bolton 2012; Osbaldiston and Mingle 2007). The milk bank also relied on ‘in-house’ donors who were resident in the hospital or whose babies were at the hospital. These donors were mainly materially impoverished patients with little education. Their family arrangements were diverse. In-house donors accounted for a small part of donations to the milk bank; indeed, there was only one in-house donor present during the eight weeks of in-hospital research.
Three staff of the milk bank and a total of seven doctors, nurses, and other hospital staff who worked in the neonatal unit were formally interviewed. Informal conversations and observations informed data collection and shaped the questions posed during interviews. Participants were medical professionals ranging from nurses to specialists, aged between twenty and sixty-five, and with varying lengths of work experience. Most of the nursing staff were black Afrikaans speakers who lived in working-class neighbourhoods of the city. Older medical specialists were mostly white, while the younger generation reflected more accurately the demographics of the country. These facts are significant, because they point to the race and class composition and dynamics of the public medical facilities that service a mostly black, working-class population.
Finally, the research included the mothers of the thirteen babies who received donated milk during the research period. The mothers of seven of babies were present in the neonatal unit while the remaining six were not present and could not be interviewed. Two residential mothers had recently experienced infant deaths (in both cases, the death of a twin), and a third was reportedly experiencing psychological distress. They were excluded from the study. The mother of a fourth recipient was discharged before she could be interviewed. The remaining mothers were interviewed. These mothers were from low-income suburbs surrounding Cape Town, were formally employed, were in their late thirties, had several older children, and lived with their husbands. None were in-house donors.
Human milk outside the mother-child dyad
A substantial literature demonstrates the complex cultural constructions and social mediations that facilitate or inhibit successful breastfeeding.[note 5] In South Africa, this discourse has historically been racialised and increasingly complicated by debates about HIV transmission, discourses that, as Van Esterik (2010) shows, differ between the global North and global South. We demonstrate these histories as they arise in relation to the ethnographic material presented here.
Even within the mother-child dyad, milk is a complex substance with varying meanings. However, when resituated from that context certain properties become more prominent. In the United States and in South Africa, it is necessary to obtain informed consent from a baby’s parents before administering donated milk, because such milk is not only food but also the bodily fluid of another person (Carroll 2014, 478). The South African National Health Act (2003) regulates the control of human bodies, blood and blood products, tissues, gametes, stem cells, DNA, etc. but, like the Human Tissues Act, does not specifically identify milk. The doctors participating in this research stated that they used regulations related to blood donation and transfer as the model for human milk trajectories, but also noted that this was unsatisfactory and that they were actively lobbying government to amend the legislation.
Despite the normative assumption of a direct and intimate relation between a mother who produces milk and the infant who consumes it, other breastfeeding histories include wet-nursing, cross-feeding, and surrogacy. While a detailed review of this literature is beyond the purview of this paper, we note that wet-nursing historically implied breastfeeding another woman’s baby on a contractual and often paid basis (Shaw 2004, 287; 2007, 442; Thorley 2008, 26). The relation between the wet-nurse and the infant’s mother was thus usually stratified by class. Cross-nursing refers to feeding another’s baby occasionally, and presumes a relationship of equality between the mothers (ibid.). In South Africa, while milk sharing among peers has been quite common, it is now strongly discouraged because of concern about the transmission of HIV from nurse to infant (Goedhals et al.2012).
While these practices challenge the dominant conception of the breastfeeding relationship as an intimate, mother-child practice (a conception that is itself socially and historically constituted), donation distinguishes the bodily practice of nursing from that of administering milk. The donating mother expresses her milk, has it collected or takes it to a milk bank and, after processing, an infant receives it by spoon, cup, syringe or bottle. While the milk donor may not be present, as we will show, this does not automatically mean that she does not experience a sense of relatedness to the recipient.
Human milk is highly valued but also seen as contaminating, transgressive, and ‘dirty’, especially in a medical context, where it is a potential bearer of pathogens (Carroll 2014; Bartle 2010), but also in a social context, particularly among women and men holding a Western, middle-class model of parenting that privileges biogenetic parenthood (Shaw 2004). The normative direction for milk is perceived to be from the mother to her baby, and concerns arise when this direction changes (Carroll 2014, 468; Shaw 2007, 440), often giving rise to repulsion (Shaw 2004, 288). Repulsion links to ideas about ‘dirt’ and ‘the abject’. Elizabeth Grosz (1994, 192) draws on Julia Kristeva’s work when defining the abject: ‘The abject is what of the body falls away from it while remaining irreducible to the subject/object and inside/outside oppositions’ (see also Shaw 2004). Mary Douglas (1966, 145) shows how bodily fluids can be seen as ‘dangerous’, marginal stuff that crosses the boundaries of the body and upsets categorical orders. Fears of infection and contamination are associated, especially in an HIV-prevalent context. Grosz (1994, 195) extends these notions by inquiring about the hierarchy or order of propriety in these bodily fluids and their different indices of control, disgust, and revulsion, as culture intervenes in the constitution of the value of the body. She argues that in contemporary Western discourse women’s bodies especially are inscribed in terms of seepage – leaking, uncontrollable, and uncontained (Grosz 1994, 203).
Potentially both sacred and contaminated/contaminating, human milk challenges categories and fundamental ideas about an individual in a bounded body. Yet this ‘danger’ is relatively contained in the sanctified mother-child dyad. (The exception here is in mother-to-child transmission of HIV, something the state has successfully diminished through the Prevention of Mother-to-Child Transmission Programme initiated in 2008.) When human milk moves beyond this ‘private’ relationship, however, the repulsion factor may be enhanced. We argue that repulsion and ‘risk’ are managed through technologies that stabilise the milk’s sacred qualities, while preventing physical and symbolic contamination, transforming the milk and its meanings in the process.
Four nodes
We identified four nodes in the donated milk’s trajectory from donor to recipient in which different dimensions of its status as food-medicine-bodily substance were foregrounded. During feeding there is a closed and highly responsive circuit between baby and mother. Milk here is generally considered a sacred substance whose function is sustenance, both bodily and emotionally. While there are risks in breastfeeding (materialised most prominently in the debates about vertical transmission of HIV and its relation to breastfeeding), the riskiness of milk is not as apparent here as in later stages of the trajectory.
Expressing and storing milk, as the milk is alienated from the dyad in which is it produced, form the first node. Immediately, concerns related to safety and security are revealed. Risk materialises as something to be managed technically. The second node of packaging is a homogenising intervention; although the milk itself is not homogenised, it is produced as uniform product. The third node consists in pasteurisation and testing, technical interventions aimed at diminishing, even eradicating, risk. After these interventions, the milk is considered ‘safe’ and its status as such is secure. Prescription is the fourth node. A technical intervention, it legitimises the dispensation of donated milk in the clinical setting. Prescription is not tailored to a specific child, but is based on an aggregate, a prediction of what a child in general needs. Through prescription, milk’s medical valence and properties are emphasised. Prescription reframes the milk’s value, foregrounding food as medicine and medicine as food. A generic product, its value lies in its capacity to sustain life.
These technological interventions foreground a shift in emphasis in donated milk from ‘food’ to ‘bodily fluid’ and then to a substance akin to ‘medicine’ that can only be offered in a medical context on prescription. Through these interventions, milk is ‘scaled up’, made generalisable through techniques that transform an idiosyncratic substance produced in a particular dyadic relation and its social formations into a substance that is more like a commodity: alienable, generic, and available to strangers. Its status is unsettled. Its meanings (temporarily) coalesce around what we describe as an axis of saving, securing, and sustaining.
Node one: Donation, expression, and storage
Among middle-class milk donors, there was a strong imperative to breastfeed, expressed in terms of an obligation to give one’s child the best start in life. Donors tend to be well read on the benefits – both emotional and physiological – of breastfeeding, and understand their donation in terms of saving the lives of (black) babies. ‘It is a bit of a schlep!’ Stella exclaimed after describing to me her routine of expressing and storing milk. Yet, she was deeply committed to donating breast milk and ‘saving lives’. In this, she was not alone. Donors consistently described their actions as altruistic. Their desire to donate was framed in terms of ‘saving lives’, something the milk bank reiterated in its advertising and recruitment programme. The milk bank was careful to explain why the mothers of recipient babies might be unable to breastfeed, but donors almost always imagined recipient babies as abandoned, and their mothers as absent, deficient, negligent, or dead. Occasionally, the mothers were understood as vulnerable, but mostly they were framed within a highly racialised discourse of the ‘bad mother’. Often, donors had not considered the mothers of recipient babies at all; their imagining was largely centred on the idea of the child in need, a child they would never meet. In fact, the mothers of most recipients were too sick to breastfeed or lived at a distance from the hospital and could not take advantage of or did not qualify for in-hospital accommodation. This was either because there was insufficient bed space or because they had other children to care for. The moral discourse of donors, which anticipates saving a child rendered worthy by maternal abandonment, was in fact the obverse of the reality in which mothers of recipient infants found themselves called to care across extended spaces, themselves the result of apartheid city spatial planning.
Expressing milk is time consuming. Some women found it discomforting: ‘You feel like a cow’, one exclaimed. While the in-house donors at the hospital were taught to express with their hands, private donors mostly used electric pumps. Unprompted, almost all donors interviewed related stories about their breast pumps. Tania described having expressed a few times while driving, using her double hands-free pump. Salma’s sister in Australia sent Salma her breast pumps, and many others were handed down through sisters and friends and returned again. (This kind of circulation can be found in all social classes, and signals an economy of exchange in a gendered circuit of intimate close friends or relatives. Indeed, in some contexts, owning a breast pump is a status marker.) Some women cut holes in their bras to make expressing easier, or tied together two single pumps to ‘speed things up’. On one afternoon Kelly and Barbara, milk bank employees, sorted through a box of pumps and pump parts that the milk bank had acquired over the years, seeing what could still be used and what should be discarded, commenting on how ‘cute’ and how ‘complex’ various pumps were. Technologies were not only part of caring practices and networks of care but also were cared for.
The milk bank discouraged people from using second-hand or sharing breast pumps, because they were a potential source of contamination. They told the donors that ‘you wouldn’t share your sister’s toothbrush’, drawing on a representation of human milk as a bodily fluid (like saliva) and explicitly invoking its ‘yuk-factor’ (Shaw 2004) and in so doing drawing on an already-established discourse of risk related to HIV infection. The message was clear: expressed milk, before freezing or pasteurisation, is risky and must be contained.
Donors expressed their milk into sterilised bottles provided by the milk bank. Although expressed milk can safely be kept at room temperature for up to twenty-four hours, it generally took most donors a few weeks to fill up the standard ‘batch’ of two litres, so they usually froze the milk at home to limit the risk of bacterial growth. An unassuming domestic appliance, the freezer, thus played an important role in the donation process. Donors kept expressed milk in their own freezer until they accumulated enough to donate. Milk bank staff who collected the milk, usually in the afternoons or evenings, kept it in their own freezers before taking it to work, where it was kept in one of two chest freezers in their office or, after pasteurisation, in the industrial freezers at the milk bank. The freezers offered a site where the everyday domestic habits of food preparation threatened to mix with the unusual practice of milk donation. Dr Muller explained: ‘I pick up the milk after work, because I have a spare big freezer now where I can store the milk overnight. I don’t really want to store it in my kitchen freezer and have the milk mix with food’. It was not clear for whose sake the food and milk should not mix, or which was considered ‘at risk’ of contamination (perhaps both). In other cases, it was unambiguous: both Lisa and Kelly from the milk bank said their partners had initially been ‘grossed out’ by having their freezers double as milk storage facilities. To them the donated milk was primarily a bodily fluid – something that they were not keen to keep close to their steaks. Abstracted from the intimacy of breastfeeding, human milk becomes ‘a repulsive other’.
We see here a shift from a discourse of ‘saving’ to one of ‘security and safety’, a shift that is consolidated through the techniques of packaging, pasteurisation, and testing that transform milk’s status from an intimate substance to one whose risk must be technically managed.
Node two: Packaging
After the donor or milk bank staff had carefully transported milk to the hospital in insulated bags, it was received by the milk bank. It was decanted from the donors’ containers and poured into uniform bottles, each labelled with the date, donor number, and batch number, and sealed. A sample from each batch was identified for post-pasteurisation testing. The unpasteurised milk was then stored in a freezer in the milk bank’s offices. Milk obtained from women known to be HIV positive, which was to be used solely for their own babies, was expressed into clearly identifiable containers (the milk bank used branded peanut butter jars) ‘to discriminate’ it from other milk, as one nurse described it, and ensure that it was administered only to those babies. Although subject to the same processes, the milk obtained from HIV-positive women was kept separately from other donor milk and went through separate pasteurisation processes to limit any potential cross-contamination. These precautions mark a symbolic as well as an actual concern with demarcating difference and producing safety.
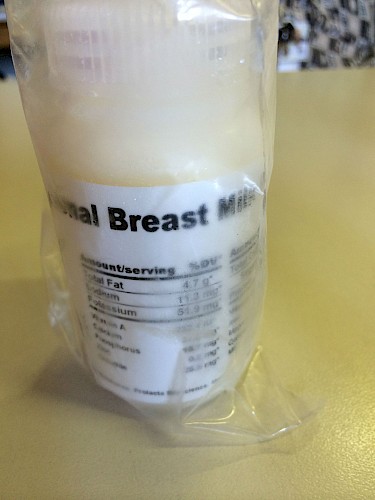
The uniform packaging of the milk aided in its abstraction in the hospital. Once decanted into neatly labelled containers, no personal trace of the donor remains; human milk is presented as a standardised product. As one of the nurses, Sister Abrahams, described it, ‘The donor is only a number’. She laughed, remembering how she had thought it strange that for months all the donor milk they received was from donor 800. She and other nurses were puzzled how one woman could have so much milk, and why it was only her milk that they were receiving. They did not know that 800 was the number given to the entire batch of the (neatly labelled) imported milk from the United States. The milk’s trajectory and origin were obscured by the single number by which it was known in this context. Unlike milk bank staff and doctors, but like the nurses, most donors and recipient caregivers were unaware that the milk bank imported and distributed donated milk from abroad.
The uniform packaging of a product renders it knowable and safe. Indeed, imported milk carries a label describing its nutritional composition and value (see figure 1).
Node three: Pasteurisation and testing
As Carroll (2014, 477) has recently shown, in hospital settings, ‘the absence of pathogens and the work of the HMB [Human Milk Bank] had transformed the milk into a highly medicalised, industrial and somewhat uniform product’. She argues that processing of the milk produced it as a legitimised medical object akin to medicine in the neonatal unit (Carroll 2014, 479). We concur. Pasteurisation was important in the South African context we researched, because it kills bacteria and viruses (including HIV) while preserving its vital nutrients (Ewaschuk et al. 2011). It thus removes risk from the milk – literally and figuratively securing its status as a safe medical object. While protocols prevented donation by HIV-positive women (although such women were encouraged to express and feed their own infants), pasteurisation and testing were essential in ensuring that milk offered by donors was free of bacterial growth and other contaminants, including HIV. Donors were not permitted to refuse the quarterly HIV test. Donors generally assumed that they were HIV negative and a number to whom we spoke were either annoyed by the HIV tests or felt they were pointless because they all ‘knew’ they were negative. Their ‘knowledge’ reflects a widespread bias in (white) middle-class South Africa that imagines the body of the HIV-afflicted person as ‘other’ and anticipates – erroneously – that middle-class, educated people do not get the virus (see also Levine and Ross 2002).
There were many different ideas about what actually happened to the milk before it was delivered to the neonatal unit. Sister Abrahams thought the milk was autoclaved. Khanyisa, the mother of a recipient, thought it was boiled. Nuraal, an in-house donor, thought it went through ‘some kind of purification process’. All of these share the idea of eliminating ‘pollutants’. Some donors expressed concerns that their milk might contain ‘bugs’ that could be harmful to recipient babies if they did not freeze the milk in time or clean the breast pump correctly.
Batches of milk, including the samples for testing, were taken from the milk bank to the clinical-sounding ‘feedprep’ room, or, as it is more familiarly known, the ‘milk kitchen’. (The changing of this term points to the issues around the symbolic separation of donated milk and food that were also apparent in the freezers of the milk collectors. As most people called it the ‘milk kitchen’, we have stuck with their usage.) Here, the ‘feeds’ for the babies in the neonatal unit were prepared. Frozen donated milk was thawed, pasteurised, and then frozen again to be distributed, and formula milk was prepared.
Despite the domesticity of its name, the milk kitchen is a sterile, technical space. Before entering, workers donned sterile clothes, hairnets, masks, gowns, and gloves. Three or four women worked in the milk kitchen every day from seven to seven, preparing formula and pasteurising and preparing human milk. One person, Ulrike, did all the pasteurising. It is labour intensive and precise work that involves heating bottles of milk, neatly stacked in wire trays in the pasteuriser, to exactly 63.5 °C to kill bacteria and viruses while retaining most of the milk’s immune properties and nutrients. Once cooled, the milk was stored in the milk kitchen’s freezer until the results of laboratory tests were received, and was distributed only after the results came back as ‘clean’. Green labels indicated that results had come back and the milk could be distributed; yellow labels identified milk whose results were still awaited. In this way, a categorical distinction between ‘unsafe’ and ‘safe’ milk was maintained visually in the freezers. Milk expressed by HIV-positive mothers for their own babies’ use was flash pasteurised and stored in a different part of the hospital.
A small sample of every pasteurised batch was sent for analysis. During the research period the milk bank changed laboratories and there had been a marked increase in the amount of bacterial growth found in the milk samples. The tests also took much longer to get back, four days instead of two. This was a problem as milk was distributed on a daily basis, so the bank did not have a lot of ‘breathing space’ to get the milk to recipients, yet could not distribute existing milk resources until the safety tests had been received. Time was always pressing. There were always babies who needed milk and there was never enough of it, yet it also took time to process the milk in a way that minimised the forms of risk discussed above. The milk bank had to balance the demands of supply and safety. Doing so put different risks in tension: minimising the risk of impurity increased the time it took to ensure safety, and increasing the time of testing introduced the risk that a baby might not get the milk on time.
Node four: Prescription
After the technological interventions described above, milk could be administered – if a doctor prescribed it. This final step was crucial to the milk’s reception in the neonatal unit, and was a technique that stabilised the donor milk as a beneficial substance akin to medicine, completely alienated from the source of its production and available for limited distribution on medical grounds.
Dr Muller explained the prescription procedure as follows:
There are forms that need to be signed, like consent forms, but we don’t want the availability of donor [milk] to undermine breastfeeding. Some women say they don’t have to express because they got donor [milk]. But donor milk is prescribed to babies who are less than 1500 grams whose moms can’t supply for whatever reason. It can be transient, so for three to four days while the mother builds up supply, or when the baby has low blood sugars and needs top-up feeds. HIV-positive moms often do not have a lot of milk. The benefits of exclusive breastfeeding are great, but [full-] term babies erode the stock quicker. There is a very limited set of criteria. I would like to expand those but the supply is limited.
The prescription process is thus marked by two inherent tensions: the fear that the availability of donor milk will have an adverse effect on establishing maternal breastfeeding, and the economic logic of supply and demand based on a model of scarcity. Although Fiona Giles (2008: 33) has proposed a ‘model of plentitude’ as a means to disrupt order and subvert scientific policing of women’s bodies, globally, there is a pervasive discourse of milk scarcity, among mothers (Scheper-Hughes 1992), in the formula industry, and in sectors that distribute donated milk. This leads to particular kinds of economic calculations, such as when Dr Jansen explained that sending a driver to retrieve milk from a mother in a nearby hospital was a cost incurred that could not be recovered through ordinary cost-recovery mechanisms, but noted, ‘It is a resource that we’re using, but milk is the sustenance of life’. Dr Taylor, another paediatrician, confirmed this:
I work with donor milk primarily in the management of our prems [premature babies], as to who we feel benefit from milk, bearing in mind that it is a limited resource. We have to make hard decisions. There are babies who we don’t give it to. I’m part of who decides who gets it. It also involves counselling the parents a lot, because when we start a baby on donor [milk] often the mom feels off the hook.[note 6] The moms need to be motivated to produce milk.
The act of prescription was an important part of the process by which an intimate bodily fluid became, in effect, both food and medicine. While much of the process of making the milk safe for consumption by preterm babies happened before it was prescribed, it was the act of prescription that really affirmed its properties, setting it apart from being ‘just food’. Prescription assured both recipient mothers and nurses that the milk was indeed safe, and more, part of a medical treatment that certain babies need.
Hygiene, risk, and safety
Questions of risk and safety are central to the ways that donated milk becomes generalizable and emerge at multiple stages in the milk’s trajectory. We explore this in detail here, drawing from Carroll’s (2014) research that demonstrates milk’s ‘paradoxical’ qualities as both therapeutic and potentially contaminating. Bartle (2010) too demonstrates the ambiguities of donor milk use in neonatal units in New Zealand, arguing that milk’s desirability nevertheless does not fully offset the uneasiness it causes in that context even among medical professionals. There are similar ambiguities in the South African context. We propose that in addition to examining the careful procedures in-house to secure the therapeutic dimensions of donated milk, it is important to consider how donors are imagined and the points at which the symbolic stability of the substance is disrupted. This is particularly the case given that fifteen years ago, in the early days of milk donation, considerable social distrust existed around whether ‘white’ milk could be given to ‘black’ babies, a framing that, while still present, seems to have become submerged in the discourse of saving lives.[note 7]
Often, the donor was often framed as being responsible for imparting the risk. This was not always true as sometimes contamination happened after donation, but it does pertain to aspects such as the transmission of viruses or other substances. Another way in which ‘risk’ is potentially materialised is in terms of the psychological effects on the mothers of recipient infants. Carroll (2014, 477) found that in the United States the ‘imagined’ presence of the donor is keenly felt and some mothers perceive this to disrupt the ‘sanctity’ of the normative mother-child dyad. While there was evidence from our study that the male partners of donating women had some similar anxieties (Waltz 2015), this was not the case for donating mothers, who envisaged their donation as an extension of their maternal roles and a fulfilment of social obligations, nor was this anxiety expressed by caregivers of recipient infants. Indeed, when asked how the milk donors are imagined, most nurses and recipient mothers said they never think about the donor. Sister Williams said: ‘It is like with adoption, you don’t want to know too much’.
By contrast, the doctors did imagine the donors; indeed, some had personal experience of donation. They all had a clear idea of what donating entailed, who the private donors were, and what happened to the milk before reaching the babies. Dr Glover had been a donor, and told me she had sometimes wondered if it was her own milk she was prescribing. Her words index the possibility that milk’s careful foregrounding as food and medicine in the hospital context might be challenged by the facts of intimacy – ‘this was my milk’, or that of known others. Dr Taylor described ‘looking out’ for milk that her friend had donated so that she could report to her friend that the milk had been received by a specific baby.
Most people felt it unnecessary to know about the donors because they trusted the prescription procedure and the processes that preceded it. For example, Sister Abrahams said, ‘I strongly believe it is from a good source. I know they are very careful’. Khanyisa, whose child was receiving donated milk, commented:
They said they check the person’s milk, see if it was healthy. Maybe they will boil it, so I don’t have to worry about disease, because the person is healthy. I would have been worried about disease if they didn’t check, but at least I know that it is good. My child is a prem so he’s very sensitive.
She said that she never wondered who the donors were: ‘I don’t think it really matters, what is important is the baby’s health, and that the person is healthy. Maybe the person will look decent but is not healthy, so it’s not important to know who the person is’.
Before her own milk came in, Petra only accepted donor milk for her baby after a doctor explained that the milk was checked first ‘or else there can be something that can make my baby sick’. She added: ‘They never told me who the milk was from, I never knew it, even today. I know only that it’s safe’. Patricia was told that donor milk comes from ‘moms who have a lot of milk and are very clean’, and that ‘they make sure there are no germs’. When asked if she ever wondered who the donors were, she said:
No, I don’t actually. … When you have enough milk, [like] moms of babies who passed away, anyone can donate, as long as it is clean. My baby never got a sickness; I would be worried if he was sick or infected. You can’t be choosy about who or why, or about colour or race. It would be wrong to say, ‘I don’t want milk from so and so’. If your child needs it, even if I know the donor and don’t like her face, if I don’t want the milk my child is going to suffer.
In these cases safety seemed to rest on notions of either health or cleanliness. The donating mother is largely left out of the picture, just as donor mothers mostly factored mothers of recipient babies out of their imaginings of the needy infant. As Petra put it: ‘I don’t want to picture the donor, as long as there is milk to help my child and the doctors say it’s safe’. Where donor mothers were considered, their characteristics as a safe source of milk were more important than individual considerations. Donors were generically imagined and represented as kind, the obverse of donor imaginings of the mothers of recipients.
This raises the question of who is considered risky and who has to accept risk. While each instance of risk must be evaluated in its own context, all evaluations fall within the broadly racialised discursive practices of South Africa, particularly (but not only) as entrenched through the HIV/AIDS pandemic, in which risk was generally understood in terms of ‘risk populations’ (particularly Africans).[note 8] As Petra put it, recipients cannot be ‘choosy’. Questions of risk and safety saturate the process of donation and receipt differently along the way. For the donors, the risk was overexpressing and endangering their supply for their own child, something the bank warned donors about regularly even while reassuring women that their supply was responsive to the demands made on it. Other risks could be managed by adhering to the milk bank and hospital’s precautions: washing hands and implements, using sterilised bottles and pumps, freezing the milk immediately after expressing. For others, such as the recipient mothers, risk was assigned and safety was something in which they had simply to trust.
Doctors related their prescription of donor milk not only to the benefits prescribed to human milk, but even more to the risks of formula. Commenting on the widespread use of formula in hospitals and clinics Dr Muller said:
What people don’t appreciate is the risk of formula. Formula can be given at any time. This is wrong, because systematic review shows that preterm babies are more likely to get sick or die when given formula, so in terms of risk parents should sign consent for formula. There is a miniscule risk of infection through donor milk, but there is more risk with formula because it is not sterile. The powder contains organisms that can grow because it is not mixed at boiling temperatures.
At the time of writing, state hospitals were transitioning from providing free formula as a consequence of implementing the state’s new Exclusive Breastfeeding Policy, which took effect on 1 April 2015. Dr Taylor described considerations in prescribing donor milk: ‘A major thing is the risk of the baby getting formula. The younger they are in terms of gestational age and days of life, and the smaller, the higher the risk’. She continued: ‘Another factor is HIV infection if the mom is high risk, so we might give donor [milk] in the early days when there is not enough to pasteurise’.
But donor milk is not without potential ‘risks’, too, as Dr Muller illustrated with the following anecdote: ‘In the last twenty-four months there was a woman with twins who didn’t have a lot of milk, so another woman in the room offered her milk. She had tested [HIV] negative before birth, but tested positive after, and after she had given the milk. So milk sharing is risky’. He further commented that ‘it is always a big exercise when milk sharing happens’. When an infant was fed by someone other than his or her mother, the woman who fed the baby and the baby itself would have to undergo HIV and hepatitis B tests. Staff found this very upsetting. Milk sharing in this particular environment was dramatic because of the underlying premise that human milk is inherently ‘dangerous’, a danger that is offset in the donor relation through pasteurisation and other mechanisms. One could argue that this assumption is ethical: practitioners must assume the worst in the best interests of the patient.
‘Risks’ of donor milk were actively addressed in the space of the hospital, and before it reached there. Donors were recruited through posters at hospitals, antenatal classes, and by word of mouth. Donors were screened through a ‘lifestyle questionnaire’ that included questions about sexual activity and the use of substances such as alcohol, nicotine, and antidepressants. They also had to undergo HIV and hepatitis B tests before their milk could enter the distribution network. Lisa told me that the milk bank was more careful in this regard with in-house donors, because they were more ‘at risk’.Her comment is linked to the facts that the hospital is a second-tier institution and therefore more likely to see women with complications in pregnancy, and to assumptions about the hospital patients, whom staff generally understood as likely to be substance dependent or HIV positive.
In the neonatal unit, staff and visitors were encouraged to wash hands. Every incubator had a bottle of disinfectant on it, and nurses and doctors sanitised their hands frequently. Nurses encouraged mothers to express into sterilised cups so that their milk could be fed directly to their babies without additional sterilisation. These practices became part of rendering the milk ‘safe’, foregrounding its medical valence. Such hygienic practices were necessary but excessive. We propose that this excess is symbolic and that it is required to create the object as conceptually stable, securing its status as medicine-food and its power to secure life.
Nurses who dealt with the milk did not always seem to have very precise understanding of what processes the milk had gone through, nor did the parents of recipient babies. Yet they all firmly believed that it was ‘safe’. For example, Sister Abrahams told me: ‘All we know is that it is tested, it is legitimate, it is safe’. Another nurse, working in the special care unit said:
I’m fine with working with donor milk. It is always pasteurised. We work with gloves and the moms are screened against any illnesses before, so the milk comes from a sterile, clean, safe, and healthy environment. So it is safe, and we just continue it being safe by using gloves and sterile cups and syringes.
The nurse’s reference to sterile gloves is telling. Nurses recalled that when gloves were first introduced they had disliked wearing them, but believed that they were an important protection against contamination. By the time of research, staff used gloves routinely and seldom thought of contamination. Although they were unable to articulate the precise nature of their earlier fears of contamination, it was likely linked to both revulsion at the administration of another’s bodily fluids and, perhaps more specifically, to fears of HIV infection, even though they all knew that donors were screened and milk was pasteurised. Their trust in gloves suggests the instability and ambiguity of prescribed milk; even though at this stage it was considered to be food-medicine, its repulsiveness was nevertheless not fully eclipsed. The emphasis on the milk’s safety – the fact that it should come from a verified source and be handled in a particular way (avoiding direct contact) – underline the awareness of the milk’s potential to bring risk. However, there were different ways of understanding potential risk in milk donation and receipt. Depending on each person’s place in the milk’s trajectory, and their relation to the milk, specific ‘risky’ aspects of processing, prescribing, distributing, and receiving donated milk stood out. For doctors, most of the risk lay in infants’ medical complications. The milk bank saw it primarily as the threat of bacterial growth but also as drugs passing into milk. Nurses seemed mainly concerned about viral transmission. Mothers of recipients had to risk trusting the supply chain. While it was widely accepted that milk donation could ‘bring risk’ with it, behaving in risky ways was not acceptable. Elaborate measures differentiated between the states of being risky and bearing risk. Bearing risk was removed as far as possible through a set of techniques, but the possibility of being risky could never be fully eliminated.
Conclusion
Milk’s characteristics of being bodily fluid, food, and medicine were differently foregrounded as it moved through different domains in its trajectory from donor to infant. Technological and medical interventions of expressing and storing, packaging, pasteurising and testing, and prescribing were nodes at which the foregrounding of different dimensions was accomplished – albeit temporarily. Technologies were implicated in the donation process from the start and feature in unexpected ways, aiding in abstracting the milk from the maternal dyad and thereby its transformation into a generalisable, generic product. Difference emerged and was consolidated as milk moved through the donation-receipt trajectory. In the process, different moral registers materialised. Meanings constellated around the metaphoric anchors of saving, security, and sustenance, each of which received different valence at different points in the cycle, and all of which fell under the broad modernist rubric of saving lives. At moments of disjuncture, such as when a nurse commented on wearing gloves while administering food that is also medicine, or a doctor envisaged encountering her own bodily fluids while prescribing milk, the multiple registers and valences of milk become particularly manifest, revealing the exuberance and volatility of the body (Grosz 1994) and the limits of efforts to contain it.
Donated milk’s simultaneous potential as treatment and as bearer of disease, along with its social and cultural possibilities of abjection and ‘saving a life’, render it semiotically unstable and productive. In this space of productive possibility and danger, technologies work to manage and guard against risk, striving to bring out milk’s positive potential. Medical authority bestows legitimacy, purifying substances through medical procedures, and temporarily stabilising meanings so that milk can become generalised, shareable with unknown others. In this way, nurses and carers of recipient babies can state that even if they did not know exactly where the milk comes from, they could trust it is healthy, disease-free, safe – in short, something you would give your baby.
Acknowledgements
Research funding from the AW Mellon Research Chair in the Anthropology of the First Thousand Days of Life at the University of Cape Town, and an NRF Innovation Grant awarded to Miriam Waltz is gratefully acknowledged. The funding bodies bear no responsibility for the data or opinions expressed here. Thanks to Linda Waldman, Diana Gibson, members of the 1000 Days research team, and anonymous reviewers. Research ethics clearance was granted by the Anthropology Ethics Committee at the University of Cape Town and the University’s Health Research Ethics Committee (HREC 705/2014). Permissions were granted by the hospital where research was conducted and the provincial Department of Health.
About the authors
Miriam Waltz completed her master’s degree in Social Anthropology at the University of Cape Town in 2015 as part of Fiona Ross’s ‘the first thousand days of life’ research group. Her research interests lie with medical anthropology, science and technology studies, and health. She is currently working as a researcher at the Sustainable Livelihoods Foundation, based in Cape Town. Address for correspondence: mhawaltz@gmail.com.
Fiona C. Ross is Professor of Anthropology at the University of Cape Town, where she currently holds an AW Mellon Research Chair on ‘the first thousand days of life’. The project is exploring how scientific knowledge, state institutions, and everyday practices are intertwined as a new knowledge field emerges. Address for correspondence: Anthropology, School of African and Gender Studies, Anthropology and Linguistics, University of Cape Town, Rondebosch, South Africa. Email: Fiona.Ross@uct.ac.za.
References
Andrew, Naomi, and Kate Harvey. 2010. ‘Infant Feeding Choices: Experience, Self-Identity and Lifestyle’. Maternal and Child Nutrition 7, no. 1: 48–60. https://doi.org/10.1111/j.1740-8709.2009.00222.x.
Avery, Melissa, Laura Duckett, and Carrie Roth Frantzich. 2000. ‘The Experience of Sexuality During Breastfeeding Among Primiparous Women’. Journal of Midwifery & Women’s Health 45, no. 3: 227–37. https://doi.org/10.1016/s1526-9523(00)00020-9.
Avishai, Orit. 2007. ‘Managing the Lactating Body: The Breast-Feeding Project and Privileged Motherhood’. Qualia Sociologica 30, no. 2: 135–52. https://doi.org/10.1007/s11133-006-9054-5.
Bartlett, Alison. 2002. ‘Breastfeeding as Headwork: Corporeal Feminism and Meanings for Breastfeeding’. Women’s Studies International Forum 25, no. 3: 373–82. https://doi.org/10.1016/s0277-5395(02)00260-1.
Bartlett, Alison. 2005. ‘Maternal Sexuality and Breastfeeding’. Sex Education 5, no. 1: 67–77. https://doi.org/10.1080/146818142000301894.
Bartlett, Alison, and Fiona Giles. 2004. ‘Meanings of Breastmilk: New Feminist Flavours’. Australian Feminist Studies 19, no. 45: 269–71. https://doi.org/10.1080/0816464042000278954.
Bartle, Carol. 2010. ‘Going with the Flow: Contemporary Discourse of Donor Breastmilk Use and Breastmilk in a Neonatal Intensive Care Setting’. In Giving Breastmilk: Body Ethics and Contemporary Breastfeeding Practices, edited by Ronda Shaw and Alison Bartlett, 122–33. Toronto: Demeter Press.
Baumslag, Naomi, and Dia Michels. 1995. Milk, Money, and Madness: The Culture and Politics of Breastfeeding. London: Bergin & Garvey.
Beck, Ulrich. 1992. Risk Society: Towards a New Modernity. London: SAGE Publications.
Blum, Linda. 1993. ‘Mothers, Babies, and Breastfeeding in Late Capitalist America: The Shifting Contexts of Feminist Theory’. Feminist Studies 19, no. 2: 290–311. https://doi.org/10.2307/3178367.
Blum, Linda. 1999. At the Breast: Ideologies of Breastfeeding and Motherhood in the Contemporary United States. Boston: Beacon.
Bolton, Verena. 2012. ‘Breast Milk and Volunteerism in South Africa’. Master’s thesis,University of Witwatersrand.
Bryant Merrill, Elizabeth. 1987. ‘Learning How to Mother: An Ethnographic Investigation of an Urban Breastfeeding Group’. Anthropology and Education Quarterly 18, no. 3: 222–40. https://doi.org/10.1525/aeq.1987.18.3.05x1134p.
Carroll, Katherine. 2014. ‘Body Dirt or Liquid Gold? How the “Safety” of Donated Breastmilk is Constructed for Use in Neonatal Intensive Care’. Social Studies of Science 44, no. 3: 466–85. https://doi.org/10.1177/0306312714521705.
Crossley, Michele. 2009. ‘Breastfeeding as a Moral Imperative: An Autoethnographic Study’. Feminism & Psychology 19, no. 1: 71–87. https://doi.org/10.1177/0959353508098620.
Coovadia, Hoosen M., Nigel C. Rollins, Ruth M. Bland, Kirsty Little, Anna Coutsoudis, Michael L. Bennish, and Marie-Louise Newell. 2007. ‘Mother-to-Child Transmission of -1 Infection During Exclusive Breastfeeding in the First 6 Months of Life: An Intervention Cohort Study’. Lancet 369, no. 9567: 1107–16. https://doi.org/10.1016/s0140-6736(07)60283-9.
Coutsoudis, Anna, Pillay Kubendran, Louise Kuhn, Elizabeth Spooner, Wei-Yann Tsai, and Hoosen M. Coovadia. 2001. ‘Method of Feeding and Transmission of HIV-1 from Mothers to Children by 15 Months of Age: Prospective Cohort Study from Durban, South Africa’. AIDS 15, no. 3: 379–87. https://doi.org/10.1097/00002030-200102160-00011.
Department of Health. 2013. Infant and Young Child Feeding Policy. Pretoria: Department of Health, South Africa. https://www.health-e.org.za/wp-content/uploads/2013/09/IYCF_Policy_2013.pdf.
Doherty, Tanya, David Sanders, Ameena Goga, and Debra Jackson. 2011. ‘Implications of the New WHO guidelines on HIV and Infant Feeding for Child Survival in South Africa’. Bulletin of the World Health Organization 89, no 1: 62–67. https://doi.org/10.2471/blt.10.079798.
Douglas, Mary. 1966. Purity and Danger: An Analysis of the Conceptions of Pollution and Taboo. London: Routledge.
Dykes, Fiona. 2005. ‘“Supply” and “Demand”: Breastfeeding as Labour’. Social Science & Medicine 60, no 10: 2283–93. https://doi.org/10.1016/j.socscimed.2004.10.002.
Else-Quest, Nicole M., Janet Shibley Hyde, and Roseanne Clark. 2003. ‘Breastfeeding, Bonding, and the Mother-Infant Relationship’. Merrill-Palmer Quarterly 49, no. 4: 495–517. https://doi.org/10.1353/mpq.2003.0020.
Ewaschuk, Julia B., Sharon Unger, Sarah Harvey, Deborah L. O’Connor, and Catherine J. Field. 2011. ‘Effects of Pasteurization on Immune Components of Milk: Implications for Feeding Preterm Infants’. Applied Physiology, Nutrition, and Metabolism 36, no. 2: 175–82. https://doi.org/10.1139/h11-008.
Galtry, Judith. 2003. ‘The Impact on Breastfeeding of Labour Market Policy and Practice in Ireland, Sweden and the USA’. Social Science and Medicine 57, no. 1: 167–77. https://doi.org/10.1016/s0277-9536(02)00372-6.
Garbes, Angela. 2015. ‘The More I Learn about Breast Milk, the More Amazed I Am’. The Stranger, 26 August. Accessed on 21 November 2016. http://www.thestranger.com/features/feature/2015/08/26/22755273/the-more-i-learn-about-breast-milk-the-more-amazed-i-am.
Giles, Fiona. 2002. ‘Fountains of Love and Loveliness: In Praise of the Dripping Wet Breast’. Journal of the Association For Research on Mothering 4, no. 1: 7–18.
Giles, Fiona. 2008. ‘The Uses of Pleasure: Reconfiguring Lactation, Sexuality and Mothering’. In Theorising and Representing Maternal Realities, edited by Marie Porter and Julie Keslo, 21–37. Newcastle, UK: Cambridge Scholars Publishing.
Goedhals, Dominique, Inéz Rousseau, Ute Halbauer, Mitta Mamabolo, and Tulio de Oliviera. 2012. ‘The Tainted Milk of Human Kindness’. The Lancet 380, no 9842: 702. https://doi.org/10.1016/s0140-6736(12)60957-x.
Golden, Janet. 1996. ‘From Commodity to Gift: Gender, Class and the Meaning of Breast Milk in the Twentieth Century’. The Historian 59, no. 1: 75-87. https://doi.org/10.1111/j.1540-6563.1996.tb00985.x.
Grosz, Elizabeth. 1994. Volatile Bodies: Towards a Corporeal Feminism. Bloomington: Indiana University Press.
Hausman, Bernice. 2004. ‘The Feminist Politics of Breastfeeding’. Australian Feminist Studies 19, no. 45: 273–85. https://doi.org/10.1080/0816464042000278963.
Hinde, Katie, and Bruce J. German. 2012. ‘Food in an Evolutionary Context: Insights from Mother’s Milk’. Journal of the Science of Food and Agriculture 92, no. 11: 2219–23. https://doi.org/10.1002/jsfa.5720.
Human Tissue Act 65. 1983. Government Gazette. Cape Town, South Africa.
Jain, S. Lochlann. 2013. Malignant: How Cancer Becomes Us. Berkeley: University of California Press.
Jansen, Jarno, Carolina de Weerth, and J. Marianne Riksen-Walraven. 2008. ‘Breastfeeding and the Mother–Infant Relationship: A Review’. Developmental Review 28, no. 4: 503–21. https://doi.org/10.1016/j.dr.2008.07.001.
Kukla, Rebecca. 2006. ‘Ethics and Ideology in Breastfeeding Advocacy Campaigns’. Hypatia 21, no. 1: 157–80. https://doi.org/10.1111/j.1527-2001.2006.tb00970.x.
Levine, Susan, and Fiona Ross. 2002. ‘Perceptions of and Attitudes to HIV/AIDS among Young Adults in Cape Town’. Social Dynamics 28, no. 1: 89–108. https://doi.org/10.1080/02533950208458724.
Lupton, Deborah. 2000. ‘“A Love/Hate Relationship”: The Ideals and Experiences of First-Time Mothers’. Journal of Sociology 36, no. 1: 50–63. https://doi.org/10.1177/144078330003600104.
Majombozi, Ziyanda. 2015. ‘Luring the Infant into Life’. Master’s thesis, University of Cape Town.
Marshall, Joyce L., Mary Godfrey, and Mary J. Renfrew. 2007. ‘Being a “Good Mother”: Managing Breastfeeding and Merging Identities’. Social Science & Medicine 65, no. 10: 2147–59. https://doi.org/10.1016/j.socscimed.2007.06.015 .
Mahon-Daly, Patricia, and Gavin Andrews. 2002. ‘Liminality and Breastfeeding: Women Negotiating Space and Two Bodies’. Health & Place 8, no. 2: 61–76. https://doi.org/10.1016/s1353-8292(01)00026-0.
Murphy, Elizabeth. 1999. ‘“Breast Is Best”: Infant Feeding Decisions and Maternal Deviance’. Sociology of Health and Illness 21, no. 2: 187–208. https://doi.org/10.1111/1467-9566.00149.
National Breastfeeding Consultative Meeting. 2011. ‘Tshwane Declaration of Support for Breastfeeding in South Africa’. South Africa Journal of Clinical Nutrition 24, no. 4: 214.
National Health Act 61. 2003. Government Gazette. Cape Town, South Africa.
Neu, Joseph, and W. Allan Walker. 2011. ‘Necrotising Enterocolitis’. New England Journal of Medicine 364, no. 3: 255–64. https://doi.org/10.1056/nejmra1005408.
Osbaldiston, Richard, and Leigh A. Mingle. 2007. ‘Characterization of Human Milk Donors’. Journal of Human Lactation 23, no. 4: 350–7. https://doi.org/10.1177/0890334407307547.
Scheper-Hughes, Nancy. 1992. Death without Weeping. The Violence of Everyday Life in Brazil. Berkeley: University of California Press.
Schmied, Virginia, and Deborah Lupton. 2001. ‘Blurring the Boundaries: Breastfeeding and Maternal Subjectivity’. Sociology of Health and Illness 23, no. 2: 234–50. https://doi.org/10.1111/1467-9566.00249.
Shaw, Rhonda. 2004. ‘The Virtues of Cross-Nursing and the “Yuk-Factor”’. Australian Feminist Studies 19, no. 45: 287–99. https://doi.org/10.1080/0816464042000278972.
Shaw, Rhonda. 2007. ‘Cross-Nursing, Ethics and Giving Breastmilk in the Contemporary Context’. Women’s Studies International Forum 30, no. 5: 439–50. https://doi.org/10.1016/j.wsif.2007.07.001.
Smith, David. 2015. ‘The Breast Milk Banks Reshaping South African Attitudes’. Guardian, 14 September. https://www.theguardian.com/global-development-professionals-network/2015/sep/14/breast-milk-banks-south-africa.
Smith, Julie P., and Mark Ellwood. 2010. ‘Feeding Patterns and Emotional Care in Breastfed Infants’. Social Indicators Research 101, no. 2: 227–31. https://doi.org/10.1007/s11205-010-9657-9.
Stearns, C. 1999. ‘Breastfeeding and the Good Maternal Body’. Gender and Society 13, no. 3: 308–25. https://doi.org/10.1177/089124399013003003.
Sutherland, Katherine. 1999. ‘Of Milk and Miracles: Nursing, the Life Drive, and Subjectivity’. Frontiers: A Journal of Women Studies 20, no. 2: 1–20. https://doi.org/10.2307/3347006.
Thairu, Lucy N., Gretel H. Pelto, Nigel C. Rollins, Ruth M. Bland, and Ncamisile Ntshangase. 2005. ‘Sociocultural Influences on Infant Feeding Decisions Among HIV-Infected Women in Rural Kwa-Zulu Natal, South Africa’. Maternal and Child Nutrition 1, no. 1: 2–10. https://doi.org/10.1111/j.1740-8709.2004.00001.x.
Taylor, Erin N., and Lora Ebert Wallace. 2012. ‘For Shame: Feminism, Breastfeeding Advocacy, and Maternal Guilt’. Hypatia 27, no. 1: 76–98. https://doi.org/10.1111/j.1527-2001.2011.01238.x.
Thorley, Virginia. 2008. ‘Sharing Breastmilk: Wet-Nursing, Cross-feeding and Milk Donations’. Breastfeeding Review: Journal of the Australian Breastfeeding Association 16, no. 1: 25–29.
UNICEF (United Nations Children’s Fund). 2005. Celebrating Innocenti 1990–2005: Achievements, Challenges and Future Imperatives. Florence, Italy: UNICEF.
Van Esterik, Penny. 2002. ‘Contemporary Trends in Infant Feeding Research’. Annual Review of Anthropology 31, no. 1: 257–78. https://doi.org/10.1146/annurev.anthro.31.040402.085428.
Van Esterik, Penny. 2010. ‘Breastfeeding and HIV/AIDS: Critical Gaps and Dangerous Intersections’. In Giving Breastmilk: Body Ethics and Contemporary Breastfeeding Practices, edited by Rhonda Shaw and Alison Bartlett, 151–62. Toronto: Demeter Press.
Wall, Glenda. 2001. ‘Moral Constructions of Motherhood in Breastfeeding Discourse’. Gender and Society 15, no. 4: 592–610. https://doi.org/10.1177/089124301015004006.
Waltz, Miriam. 2013. ‘“Making a Person”: Experiences of Breastfeeding among Middle-class Women in Cape Town, South Africa’. Bachelor of Social Science Honours Thesis, University of Cape Town.
Waltz, Miriam. 2014. ‘Milk and Management: Breastfeeding as a Project’. Anthropology Southern Africa 37, no. 1-2: 42–49. https://doi.org/10.1080/23323256.2014.940188.
Waltz, Miriam. 2015. ‘Milk, Meaning and Morality: Tracing Donated Breast Milk from Donor to Baby’. Master’s thesis, University of Cape Town.
WHO (World Health Organization). 2011. ‘Guidelines on HIV and Infant Feeding for Child Survival in South Africa’. Bulletin of the World Health Organization 89: 62–67.
World Bank. 2016. ‘Mortality Rate, under-5 (per 1,000 live births). http://data.worldbank.org/indicator/SH.DYN.MORT?locations=ZA.
Endnotes
1 Back
All names are pseudonyms. The hospital at which the research took place will be referred to as ‘the hospital’ and the milk bank as ‘the milk bank’ to protect the privacy of participants.
2 Back
Following Penny van Estrik (2015), we refer throughout to ‘human milk’, ‘donated milk’, or simply ‘milk’, rather than the term ‘breast milk’, although this term is commonly used in South Africa. As Van Estrik notes, we do not refer to cow’s milk as udder milk; ‘Why stress the container over the species?’ she wonders (Van Estrik 2015, xv).
3 Back
‘Kangaroo care’ involves facilitating as much direct skin-to-skin contact between infant and carer as possible to stimulate infant well-being. Kangaroo-care medical facilities offer residential services to carers.
4 Back
The Human Tissue Act (No 65 of 1983) stipulates that only authorised institutions and persons are allowed to receive payment for human tissue. Breast milk is not mentioned in that act but falls under ‘tissue’: ‘Any human tissue, including any flesh, bone, organ, gland or bodily fluid, but excluding any blood or gamete’. While the act has been repealed and material dealing with tissue transfer and donation replaced by the National Health Act of 2003, human milk remains excluded from specific mention. We are currently preparing a paper on the economic implications of milk circulation.
5 Back
It is beyond the purview of the current article to review this material; readers are referred to, among others, Van Esterik 2002; Shaw and Bartlett; 2010; Bartlett 2002; Bartlett 2005; Bartlett and Giles 2004; Blum 1993; Dykes 2005; Galtry 2003; Hausman 2004; Crossley 2009; Kukla 2006; Lupton 2000; Marshall et al. 2007; Murphy 1999; Stearns 1999; Taylor and Wallace 2012; Wall 2001; Baumslag and Michels 1995; Blum 1999; Bryant-Merrill 1987; Schmied and Lupton 2001; Sutherland 1999; Andrew and Harvey 2010; Avishai 2007; Mahon-Daly and Andrews 2002; Avery, Duckett, and Frantzich 2000; Giles 2002; Golden 1996; Majombozi 2015.
6 Back
This was a common idea among medical practitioners but it is unfair to the mothers in question as it is difficult to establish breastfeeding if it is not initiated early and well supported.
7 Back
Anna Coutsoudis, who established South Africa’s first milk bank, for example, remembers ‘At the beginning I heard nurses asking, how can you bring milk from a white woman for a black baby?’ (Smith 2015).
8 Back
Careful research and public work shifted the discourse to ‘risk behaviours’ and there seems now to be a swing back to identifying populations at risk (such as long-distance haulage drivers). This offers an interesting angle into thinking about Ulrich Beck’s (1992) idea of ‘risk society’. As Lochlann Jain (2013) has shown, risk and riskiness need to be understood in terms of the discourses that produce them and the bodies of knowledge that shape these.