Opening up ‘fever’, closing down medicines
Algorithms as blueprints for global health in an era of antimicrobial resistance
—
Abstract
The last decade has seen an increasing drive for more targeted use of medicines to address health challenges in low- and middle-income countries (LMICs), which is both a response and corrective to previous interventions premised upon blanket, presumptive coverage. The drive to rationalise the use of medicines partially reflects the recognition that such blanket coverage masks other conditions that may be going untreated. Increasingly, however, it also speaks to anxieties that medicines will lose their effectiveness in the future due to resistance from indiscriminate use in the present (O’Neill 2016; WHO 2015). Antimicrobial resistance (AMR) has cast into the spotlight an unsustainable reliance on antimicrobial medicines as well as the numerous social, political, and economic factors that have enabled medicines to flow unabated. Wide-ranging interventions to address AMR include awareness and behaviour-change programmes, revised clinical guidelines, improved surveillance capacity, stewardship programmes, and market stimulation for new drugs and point-of-care diagnostics. All are oriented towards rationalising the use of medicines and preventing a return to what some have called the ‘medical dark ages’ (Walsh 2014).
Anthropologists and social scientists have responded to this surge of activity by critically interrogating the discursive framing of AMR and the imperatives that it foregrounds and obscures (Brown and Nettleton 2017; Chandler, Hutchinson, and Hutchison 2016; Chandler 2019; Hutchison, Knight et al. 2018). Of particular concern is that the drivers of AMR are disproportionately located within the problematic ‘behaviour’ of providers and patients – meaning their propensity to ‘misuse’ and ‘overuse’ antimicrobials – at the expense of a broader understanding of the persisting inequalities that lead people to both rely upon and ‘irrationally’ use antimicrobial medicines (Chandler, Hutchinson, and Hutchison 2016; Haenssgen, Charoenboon, and Khine Zaw 2018). While AMR presents good cause to reflect upon these inequalities and challenge the pharmaceuticalised forms of care that have been especially detrimental in LMICs (Biehl 2007; Biehl and Petryna 2013; Nguyen 2010), Hutchinson (2016) observes this has not occurred and in fact AMR discourse has actually reinforced the centrality of medicines. As the discussion of medicines expands, Hutchinson (2016, 22) notes, ‘vulnerable people seem to disappear and instead vulnerable medicines take their place’. The outcome of such discursive framing is that the imperative to ration appears a common-sensical and technical concern rather than a political one (Ferguson 1990; Hutchinson 2016), obscuring inequalities in access to medicines and care that AMR seems poised to create and exacerbate.
In this article, we examine the role that ‘global’ clinical algorithms have played in creating, transporting, and normalising the centrality of medicines to care in resource-limited settings, and how they are now being reworked to ‘rationalise’ their use. We focus on a guideline called the ‘Integrated Management of Childhood Illness’ (IMCI), which was developed by the World Health Organisation (WHO) and UNICEF in the mid-1990s as a low-resource tool for the classification and treatment of common childhood illnesses and has since been widely adopted.[note 1] Drawing on Bowker and Star’s (1999) work on classification systems as both analytic frame and method, we position the IMCI algorithm as a blueprint for global health that categorises illnesses, patients and care in particular ways and with which numerous elements of health care infrastructure are built. Originally scripted with the somewhat conflicting imperatives to provide integrated care and preserve ‘bare life’ (Agamben 1995), medicines have long been the tail wagging the dog in the algorithm, with available medicines determining which illnesses are made visible and invisible. We contend that, as global health priorities are shifting away from providing blanket, presumptive coverage in light of concerns around AMR, the centrality of medicines in the algorithm has not fundamentally shifted or been supplanted. To the contrary, it has only faded further into the woodwork as new diagnostic technologies and classificatory (in)visibilities have been added. This revised script, we argue, implicitly renders some people ‘deserving’ of these precious commodities and others not, and raises the unsettling possibility that an increasingly high-tech but ‘empty’ form of pharmaceuticalised care is being worked into the infrastructure of weak health systems, with the blame for ‘overuse’ of medicines deflected onto the ‘irrational’ behaviours of end-users.
To illustrate our argument, we offer a classificatory ‘reading’ (Bowker and Star 1999) of the IMCI’s handling of a seemingly simple and mundane but in fact broad and ambiguous symptom: ‘fever’ (Beisel et al. 2016; Chandler et al. 2012; Kamat 2006). Alongside ‘cough or difficult breathing’ and ‘diarrhoea’, ‘fever’ is one of the key starting points for the classification of common illnesses. We pay particular attention to the place of ‘nonmalarial fever’, which has shifted from a residual, ‘other’ category in the shadow of malaria to an increasingly legible category following the insertion of rapid diagnostic tests (RDTs) into the algorithm and an ensuing spike in antibiotic use. Exploring the interplay among illness categories, infrastructure, medicines, and diagnostics, we aim to show, firstly, that antimalarial and antibiotic medicines have played a large role in the shape and content of the algorithm and, secondly, that recent attempts to open up and refine the fever category can also be read as an exercise in closing down medicine options. This, in turn, begs questions for further research on how clinicians, patients, and other actors are navigating these shifting scripts at a time when experience and clinical acumen are being increasingly labelled ‘irrational’ and medicines are being placed on ever-higher shelves.
Approach
Guiding our analysis is a preoccupation with classification. How, as Canguilhem (1966) would ask, does a symptom or biological state come to be classified as ‘normal’ or ‘pathological’? How do illness categories relate to one another, and how are they organised, systematised, and prioritised? Anthropology has a longstanding preoccupation with how people classify their everyday worlds, with Durkheim and Mauss’s Primitive Classification ([1902] 1969) and Levi Strauss’s Elementary Structures of Kinship (1949)beinginfluential examples of how material, social, and cosmic worlds could be mapped in form and function through classificatory systems. Medical anthropology emerged through ethnographic work that characterised the place of medical beliefs in non-Western cultural systems, for example in Evans-Pritchard’s Witchcraft, Oracles and Magic (1937). Illness classification studies remained influential as medical anthropology crystallised as a subdiscipline and was theoretically enriched as anthropologists drew distinctions between personalistic and naturalistic causes (Foster 1976) and denotive and connotative aspects of illness (Good 1977; Nichter 1996), and came to view health care itself as a cultural system to be read through ascertaining classification models (Kleinman 1980). Classificatory approaches indeed characterised medical anthropology’s emergent role in international health, with local categories for diarrhoeal diseases, respiratory infections, and malaria used to inform health messaging and interventions (Nichter and Nichter 1996; Nichter 2008).
With medical anthropologists moving away from viewing health care as a cultural system (as part of the more general move from structuralism to post-structuralism in the social sciences and humanities), illness classification studies have become less commonplace within anthropology. Yet, classificatory processes remain central to governing health and have continued to be studied, often implicitly, as medical anthropologists turned attention towards unequal distributions of power and resources (Baer, Singer, and Susser 1997), the shifting biopolitics of global health (Biehl and Petryna 2013; Nguyen 2010; Pfeiffer and Nichter 2008; Prince and Marsland 2015), and the recent rise of point-of-care diagnostics (Chandler et al. 2012; Beisel et al. 2016; Engel et al. 2017; Street 2018). One might read from this trajectory an inversion in the classificatory processes studied by anthropologists: from describing how people classify illness and the worlds around them to how people are increasingly classified, organised, and governed through the very diseases that afflict them. Bringing classification work explicitly back within the purview of anthropological analysis, in this article we draw upon Bowker and Star’s influential work Classification and Its Consequences (1999; see also Star 1999). This text has contributed to the ‘infrastructural turn’ in anthropology (Bruun-Jensen and Winthereik 2013; Street 2014; Umlauf and Park 2017), and we similarly find it useful here to understand the work that the IMCI is doing in the making of global health imperatives and the reinvigoration of discourses around (ir)rationality.
Bowker and Star (1999) examine how people’s everyday practices of classifying the world around them increasingly rub up against large-scale classification systems (among their examples are the International Classification of Diseases (ICD) and apartheid’s racial classifications). As they become embedded as infrastructure, these systems tend to fade into the woodwork and become naturalised but are nonetheless social and political artefacts that reflect the contexts of their production. Bowker and Star (1999, 68–72) demonstrate that while classification systems strive for ‘objectivity’ and ‘completion’, they are at the same time highly pragmatic by necessity. For in order to be transportable they must contain a manageable number of categories, which means excluding certain ones in favour of others (they note that the ICD, for instance, has historically prioritised diseases that threaten public health over rarer, noncontagious conditions). Such pragmatism, we suggest, is especially evident when systems such as the IMCI are designed for use in resource-limited settings, in which difficult choices must be made over where to direct scarce resources.
Imperatives, politics, and knowledge shift over time, however. Thus, as Bowker and Star (1999) show, classification systems must be continually reworked to maintain their legitimacy, including both formal work performed on the systems by designers and informal work performed by end-users to render these systems operable in context (for example, ‘workarounds’). Crucially, classification work can never start from scratch and must contend with what is there already, including the legacies of classifications past that constrain what is possible and even imaginable in the present. To study classification systems and bring to the fore their (shifting) social and political underpinnings, Bowker and Star (1999, 55) propose closely ‘reading’ them: ‘[t]he trick is to read the classifications themselves, restoring the narratives of conflict and compromise as we do so’. A meticulous classificatory ‘reading’ is the methodology that we have used to analyse the imperatives undergirding the IMCI algorithm and changes over time. In particular, we examined the algorithm before and after the insertion of a malaria RDT and the classificatory consequences of this technological addition. We support our classificatory reading with a review of relevant public health literature and policy materials surrounding the IMCI algorithm, as well as ethnographic literature from malaria-endemic regions in Africa and Asia. Having contributed to the ethnographic literature ourselves, writing this article has necessitated a reflexive rereading of our own work through the lens of classification. Our aim, ultimately, is to position the algorithm as both a revealing medium through which to view the shifting imperatives of global health – including medical anthropological responses to these shifts – and also as an actor in the Latourian sense (Latour 1979) with material force in the world: a classificatory blueprint that organises thought and action across multiple levels of scale and communities of practice.
Blueprints for global health
The IMCI strategy was conceived in the 1990s following an increasing recognition among child health experts that sick children in resource-limited settings usually presented at health care facilities with multiple illnesses (Weber et al. 1997; Wolfheim 1998). Emerging out of a merging of two disease-specific programmes on diarrhoea and acute respiratory infections (Wolfheim 1998), and a concern to address a ‘bottom-up’ need in the face of historical ‘top-down’, siloed disease-control strategies (Mills 1983), the IMCI strategy was intended to aid front-line, nonphysician health workers in distinguishing comorbidities in children. There was, on the one hand, a vision written into the IMCI strategy for a holistic approach to the management of childhood illnesses, one in which children ‘should not be regarded as a case of some particular disease, but as a sick person’ (Wolfheim 1998, 178). On the other hand, it was unequivocally designed as a low-resource tool that was calibrated to ensure child survival (Jacobs and Merson 2018). The IMCI has historically prioritised treatment for the most common causes of mortality in children – malaria, pneumonia, and diarrhoea – while referring more complex cases to higher-level facilities where the necessary resources and expertise are (at least in theory) available (WHO and UNICEF 1999).
Undergirding the IMCI strategy is a generic algorithm (Perkins et al. 1997), made available and easily accessible in the form of a booklet. While IMCI has a number of goals – including improving the skills of health workers, altering the health practices of families and communities, and strengthening overall health systems – all are built upon the foundations of the algorithm (WHO 2017). This algorithm, as one WHO (2002, 1) guideline states, ‘will classify children in ways that will lead to correct action’. The explicit intention to take the task of classification out of the hands of health workers and rely instead on globally accepted standards indexes the broader drive towards evidence-based medicine (EBM). The rise of EBM and its tensions with clinical acumen have received considerable attention by social scientists and historians (see, for example, Armstrong 2002; Chandler et al. 2008; Chandler et al. 2012; Dixon and Tameris 2019; Human 2011; Lambert 2006; Marks 1997; Timmermans and Berg 2003). What arguably makes IMCI distinctive among standardised approaches to diagnosis is that, for all its influence and reach, it is based upon very few clinical signs – little more than ‘fever’, ‘diarrhoea’, and ‘cough or difficult breathing’. In fact, as we observe below, the range of categories deployed in the algorithm is highly simple and pragmatic, using a combination of disease terms (such as ‘malaria’ and ‘pneumonia’) and more syndromic classifications (such as ‘very severe febrile disease’) that cover a range of conditions (Rowe et al. 1999). Pared down to a very limited number of basic categories, each one is attributed huge weight in the allocation of treatment and resources – and, conversely, the withholding of them.
Given the ‘normal emergency’ (Feierman 2011, 172) of public service provision in the settings for which IMCI was designed, ‘adherence’ to such algorithms is often only partial in practice (discussed further below). Nonetheless, it is important to consider how foundational IMCI is to the organisation of paediatric care and to understand the work that it does in the infrastructure of health systems. To give a sense of this, an IMCI strategy timeline (figure 1) – published in WHO’s 2017 global survey report – highlights the numerous developments that have been built upon the foundations of the algorithm since the 1990s, which have gradually expanded from primary health care to community-based care and hospital-level care. Moreover, the algorithm’s influence goes beyond that which is explicitly part of the IMCI strategy: it has had significant effects for procurement and accounting systems, disease reporting mechanisms, funding streams, and more. Moreover, the generic IMCI algorithm has not only given rise to numerous local adaptations but also to more recent and novel IMCI-based electronic algorithms that are being imagined as replacements for the paper-based booklets that have historically been used (Keitel and D’Acremont 2018). We contend that the IMCI algorithm is therefore more than a guideline for health workers: it can be conceptualised as a blueprint for global health, one upon which the infrastructure of frontline health care is built and arranged to reinscribe existing practices.
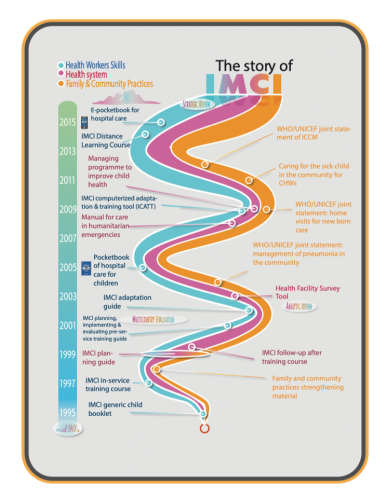 Figure 1. ‘The story of IMCI’. Reprinted from Integrated Management of Childhood Illness Global Survey Report, WHO, page 11, copyright 2017. Accessed 1 October 2018. http://www.who.int/maternal_child_adolescent/documents/imci-global-survey-report/en/.
Figure 1. ‘The story of IMCI’. Reprinted from Integrated Management of Childhood Illness Global Survey Report, WHO, page 11, copyright 2017. Accessed 1 October 2018. http://www.who.int/maternal_child_adolescent/documents/imci-global-survey-report/en/.Yet, we would also contend that the care infrastructure that has emerged is not integrated and holistic – as was the explicit intention underlying the IMCI strategy – but rather disjointed and fragmented. As Jacobs and Merson (2018, 1) argue in a recent appraisal of the IMCI strategy in the BMJ, ‘attention has moved to vertical child health areas such as immunisation and/or specific communicable diseases. Thus, IMCI’s uniquely holistic view of child health has arguably been lost’. What we would add here is that, while the IMCI strategy might have been designed to be holistic, the fact that the algorithm is and always has been effectively a tool for distributing (and withholding) medicines lends itself to precisely the narrow, disease-specific approach that it sought to avoid.
The world of siloed, disease-specific interventions that has come to characterise ‘global health’ has long been studied and critiqued by medical anthropologists. Some have highlighted the novel sovereignties, subjectivities, and citizenships brought about by research and interventions into ‘big’ diseases like HIV and AIDS, TB, and malaria (for example, Biehl and Petryna 2013; Nguyen 2010; Pfeiffer and Nichter 2008; Prince and Marsland 2015), while others have focused on diseases that by contrast have been neglected and underfunded (for example, Allen and Parker 2011; Herrick 2017; Livingstone 2012). Sitting somewhat between these two approaches, our contribution here is to focus on the work that seemingly ‘simple’ and ‘mundane’ categories like fever have been made to perform in foregrounding and reproducing the visibility of some diseases over others.
A classificatory reading of ‘fever’
Our critical reading of the IMCI algorithm evolved from previous ethnographic fieldwork observing these guidelines being put (or not) into practice in health care settings in Tanzania and Uganda, a decade of engagement in the emerging field of nonmalarial febrile illness, and our on-going research on the use of antimicrobial medicines for nonmalarial febrile illnesses in Zimbabwe, Malawi, and Myanmar. The current analysis involved meticulous desk reviews of electronic copies of the booklet available from the WHO’s website (WHO and UNICEF 2008, 2014) and related public health literature. This is of course rather different to the way that the algorithm features in everyday rhythms of public service provision. As we have noticed in our own past fieldwork, the algorithm is rarely explicitly ‘read’ but rather appears as tacit knowledge gained during training, with hard copies consulted when necessary (although increasingly run through electronic devices [Keitel and D’Acremont 2018]). Whatever form the algorithm takes, it is intended to systematically lead health care workers through a series of steps that must be taken for each child who enters the facility, from illness classification, to treatment, to possible referral. After checking for danger signs (which entail immediate referral), the algorithm progressively goes through what to do if a child has a cough or difficulty breathing, then diarrhoea, then fever.
Fever is one of the most common symptoms leading people to seek health care, and it is the starting point for the management of many illnesses (WHO 1993). It is estimated that children under five years old in malaria-endemic areas experience between two and nine fevers per year and that three-quarters of children presenting at health centres are febrile (Unitaid 2018). The category of ‘fever’ can be characterised by both its utility and ambiguity for providers and patients (Helman 2007). On the one hand, fever is an easily detectable indicator of ill health, which can not only be recognised qualitatively as an abnormally ‘hot body’ but also be rendered ‘objectively’ through the technology of thermometers (Ogren 1990; Thgawa 1985). On the other hand, thermometers are not always available in the settings where IMCI is implemented and, moreover, fever can manifest in multiple ways, including shivering and chills, sweating, muscle ache, headache, and general weakness, and can be caused by anything from infections to chronic conditions to drug interactions. As a recent Unitaid report explains: ‘Fever is an incredibly broad category, encompassing multiple clinical conditions and affecting billions of people, with hundreds of causative agents, and several possible diagnostic approaches’ (Unitaid 2018, 22). Because of its broadness and ambiguity, when it comes to an algorithm like IMCI that relies heavily on clinical signs, choices must be made regarding where to point ‘fever’ to allocate available medicines. To begin to show this, we turn to a segment from the 2008 edition of the IMCI chart booklet, which represents the era of malarial medicine before wide-scale implementation of RDTs.
Fever = (anti)malaria(ls)
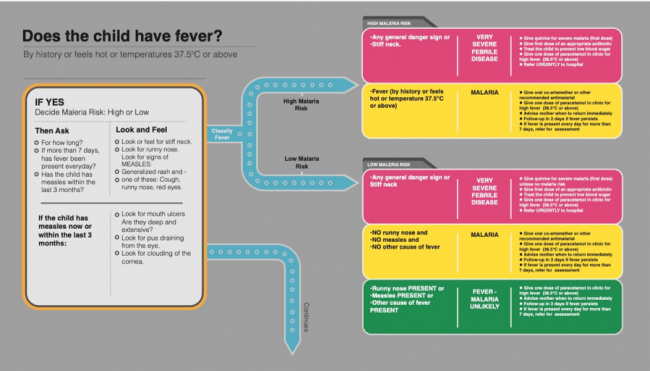 Figure 2. Fever algorithm from the 2008 IMCI chart booklet.. Reprinted from Integrated Management of Childhood Illness Chart Booklet, 2nd ed., WHO and UNICEF, page 4, copyright 2008. Accessed 1 October 2018. https://apps.who.int/iris/bitstream/handle/10665/43993/9789241597289_eng.pdf.
Figure 2. Fever algorithm from the 2008 IMCI chart booklet.. Reprinted from Integrated Management of Childhood Illness Chart Booklet, 2nd ed., WHO and UNICEF, page 4, copyright 2008. Accessed 1 October 2018. https://apps.who.int/iris/bitstream/handle/10665/43993/9789241597289_eng.pdf.Working from left to right – as might a health worker – the first step in this part of the algorithm is to determine whether the patient has a fever. This initial classification can be made on the basis of multiple kinds of evidence. The quantitative reading of body temperature produced by thermometers is prioritised in cases where such ‘objective’ technologies are available. However, the utility of the algorithm is that it also recognises the more hands-on, qualitative assessment of whether the patient ‘feels hot’, the recent history of fever via the testimony of patients or carers (Banco and Veltri 1983), and previous clinical encounters. A determination of fever is therefore constructed as a relational and dialogical process, premised upon a combination of available technologies (thermometers), clinical acumen, phenomenology, and communicative practices across professional and popular sectors (Kleinman 1980). There is hence room in the clinical encounter for popular constructions of fever and its significance; for instance, views about the progression of ‘mild’ to ‘severe’ fever (which may not follow biomedically defined cut-offs like 37.5 degrees) or the notion of ‘inside fever’ – documented in numerous settings in Africa and Asia – that is undetectable by touching the body or by a thermometer (see Nichter 2008, 31).
At the same time, the classificatory outcome of something being called ‘fever’ leaves considerably less room for interpretation. In areas of high malaria risk, there are two possibilities, represented by the red and yellow boxes respectively: ‘very severe febrile disease’ and ‘malaria’. Whilst a classification of very severe febrile disease necessitates antibiotics and urgent referral to hospital, both possibilities entail immediate antimalarial treatment without diagnostics. In other words, the identification of fever in areas of high malaria risk is equivalent to a presumptive diagnosis of malaria. ‘Fever equals malaria’, as Beisel and colleagues (2016, 1) have phrased this. Notably, in areas with a low malaria risk, a third classificatory possibility is included, represented by a more benign-looking green and the words: ‘fever – malaria unlikely’ – one of the IMCI’s many pragmatic syndromic classifications. However, the very fact that this residual ‘other’ category is defined in the negative – in terms what it probably is not – only further reinforces the pervasiveness of the fever-malaria equivalence.
To suggest that not all cases of fever are malaria is certainly not controversial. The insertion of a classificatory equivalence between fever and malaria reflects a choice that was convenient in the earlier days of malaria control to prioritise malaria treatment above other causes of fever but that gradually solidified into a pervasive malaria discourse, especially in Africa. The limitations of this equivalence were well-known when the IMCI strategy was established (see, for example, Rowe et al. 1999; Chandramohan, Jaffar, and Greenwood 2002) and have been critically interrogated in subsequent epidemiological research (see, for example, Chandler et al. 2017; Hopkins, Asiimwe, and Bell 2009; Hopkins et al. 2017; Reyburn et al. 2004; Reyburn et al. 2007). Anthropologists have situated this equivalence within the political and epistemological contestations of global health (Chandler et al. 2008; Chandler and Beisel 2017; Ribera and Hausmann-Muela 2011). What becomes clearer in attending to how clinical algorithms visualise, rank order, and colour code is just how hard it becomes to think and act outside of their categories and how subtly they embed and naturalise the epidemiological realities they represent. Above, we began to position the IMCI algorithm as a blueprint for global health; to this we might now more specifically highlight the classificatory melding of fever and malaria. Upon this nexus, staff are trained, medicines are procured, patients are classified and treated, numbers are reported; in fact, the success of malaria-control efforts have often been evaluated by the hours between fever onset and access to antimalarial medicines.
Ethnographic studies in malaria-endemic areas have demonstrated how the fever-malaria equivalence has become embedded in infrastructure, discourse, and even language itself. Two examples from Tanzania are especially informative. Chandler and colleagues (2008) observed that the organisational infrastructure of two Tanzanian hospitals made malaria far easier to diagnose than other illnesses, with support for alternative diagnoses (such as meningitis) almost completely absent. Clinicians also experienced pressure from many sources to prescribe antimalarials, including public health messages, peers, and patients, the last of whom were perceived to complain if they left empty-handed. One might conjecture that in the face of such pressures, the IMCI’s pragmatic category of ‘very severe febrile disease’ was highly slanted towards malaria in practice. Working in periurban Tanzania, Kamat (2006) observed important semantic overlays between ‘fever’ and ‘malaria’ among health centre staff when communicating with mothers. As Kamat (2006, 2950) writes, ‘in the local context, the term homa is often used interchangeably to refer to ordinary fever, malarial fever, and a range of other fevers’. Because health facility staff used the KiSwahili term ‘homa’ so interchangeably, this resulted in the double burden of some mothers strategically reporting febrile episodes in order to be prescribed antimalarials, and others classifying genuine malaria episodes as ‘only an ordinary fever’ (Kamat 2006, 2947). Other studies, too, identify overlaps among ‘fever’, ‘malaria’, and other illness categories both natural and personalistic (Hausmann-Muela et al. 1998; Kamat 2008; Langwick 2007; Williams and Jones 2004). Taken together, the ethnographic record shows the traction of ‘fever equals malaria’ in settings where care has largely been reduced to the blanket, presumptive coverage of medicines.
Malaria RDTs: from antimalarials to antibiotics
A decisive shift, however, occurred in 2010: the WHO changed its guidelines to restrict the prescription of artemisinin-based combination therapy (ACT) to confirmed malaria cases (WHO 2010; see also Chandler et al. 2012). This followed from a number of interlinked factors, including growing evidence of and concerns over the ‘overuse’ of antimalarial medicines; the increasing unpopularity of blanket, presumptive approaches to prescribing medicines for being economically inefficient and fuelling AMR; the declining (although now plateauing) incidence of malaria in Africa, and the development of an RDT for malaria. The debates surrounding the revision were complex and protracted, with several experts contesting the change, but the decision – and the shifting imperatives it reflects – eventually prevailed and were inserted into the IMCI algorithm. To highlight the classificatory consequences, Figure 3 presents the 2014 iteration of the algorithm.
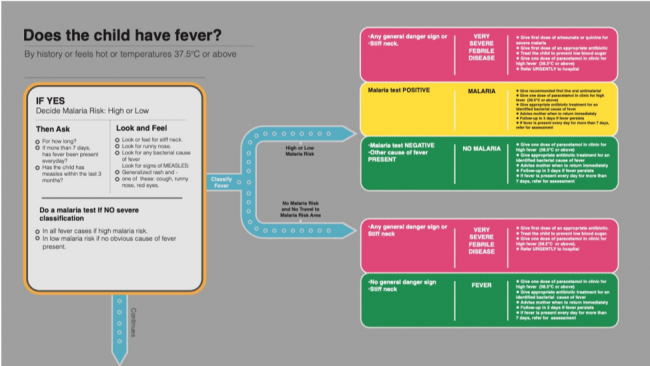 Figure 3. Fever algorithm from the 2014 IMCI chart booklet. Reprinted from Integrated Management of Childhood Illness Chart Booklet, 3rd ed., WHO and UNICEF, page 4, copyright 2014. Accessed 1 October 2018. https://apps.who.int/iris/bitstream/handle/10665/104772/9789241506823_Chartbook_eng.pdf.
Figure 3. Fever algorithm from the 2014 IMCI chart booklet. Reprinted from Integrated Management of Childhood Illness Chart Booklet, 3rd ed., WHO and UNICEF, page 4, copyright 2014. Accessed 1 October 2018. https://apps.who.int/iris/bitstream/handle/10665/104772/9789241506823_Chartbook_eng.pdf.A reading of this new iteration suggests that the basic shape and structure of the algorithm remains the same – still largely built around the diagnosis and treatment of malaria. The health worker is asked to determine whether the patient has a fever (via thermometer, history/testimony, or qualitative assessment), to perform a clinical assessment, and to classify the illness based on the risk of malaria. But they are now also instructed to ‘do a malaria test’. This instruction, while written into the script quite inconspicuously, has profound classificatory knock-on effects and is accompanied by a range of novel practices and classificatory outcomes that are layered over and redirect the previous ones. Firstly, there is no longer a bifurcation between high- and low-risk malaria areas; such is the apparent certainty generated by the malaria RDT that this epidemiological distinction is no longer meaningful for the diagnosis of malaria. In fact, with the exception of severe febrile disease, antimalarials are prescribed (or denied) entirely on the basis of the RDT. Whereas in the previous iteration of the algorithm, experience and clinical observations were direct pathways to antimalarials, the new version short-circuits the empirical observation in favour of the test.
Ethnographic studies suggest that malaria RDTs have had profound effects as they have been implemented, from governmental policy and practices attempting to ‘rationalise’ the use of ACTs (Chandler et al. 2012) to retraining health workers away from ‘old school’ empirical diagnosis toward ‘modern’ testing practices (Chandler, Hutchinson, and Hutchinson 2016; Hutchinson, Reyburn et al. 2015), to interventions beyond the formal sector including the rollout of RDTs in informal drug shops, a move that grants such shops greater recognition and legitimacy within the health system (Hutchinson, Hutchinson et al. 2017). Burchett and colleagues (2017) suggest that acceptance and uptake of RDTs can, under certain circumstances, be high. However, this requires either ‘contexts where integrating tests into practice already makes sense, or tailored interventions to encourage this’ (Burchett et al. 2017, 11). The flip side of this equation is that, despite the rhetoric of RDTs as generating greater certainty, in practice the tests often have the opposite effect of producing uncertainty. RDTs are often unavailable (Umlauf and Park 2017), a possibility accounted for in the IMCI algorithm with the instruction (written in a footnote) for health workers to presumptively treat fever with antimalarials in such instances. But also, negative results must contend with the entrenched practice of empirical malaria diagnosis, reflected in the prescription routines of health workers and the expectations of patients (Chandler et al. 2008; Chandler et al. 2012), and indeed with the meanings of ‘fever’ itself, as suggested by the findings of Kamat (2006) above. With traces of the old classificatory regime lingering in the algorithm and the infrastructures into which it is woven, RDTs have not simply overridden the practice of empirical treatment.
Overall, the RDT appears to have led to a decline in the prescription of antimalarials (Hopkins et al. 2017). However, it has also had an unintended effect on prescription practices, one that can be located in the shifting place of ‘nonmalarial fever’. A previously residual, ‘other’ category, lingering in the shadow of malaria, nonmalarial fever is still defined in the negative and as a broad syndromic umbrella, but it now features prominently in the post-RDT algorithm, both in low- and high-risk malaria areas. Perhaps the most notable feature of this open and ambiguous category is that, whereas before the insertion of the malaria RDT the only treatment advised at the point of care was paracetamol, the box is now occupied by antibiotics. This corresponds to new clinical guidance (on the left side of the algorithm) to ‘look for any bacterial cause of fever’, for which more specific guidance is provided in another footnote. It is against this backdrop that studies have found that, while the prescription of antimalarial drugs has decreased since the introduction of the RDT, the prescription of antibiotics has risen in turn (Batwala, Magnussen, and Nuwaha 2011; Hopkins et al. 2017; Johanssen et al. 2017).
Although some rise in antibiotic use was expected given that many nonmalarial fevers were previously (mis)classified as malaria, the concern is that, like antimalarials before it, this spike reflects increasing ‘irrational’ use of antibiotics. Attention has thus been drawn to the paucity of guidance on whether and which antibiotic(s) to prescribe currently scripted into the algorithm: ‘give appropriate antibiotic treatment for an identified bacterial cause of fever’ (and to refer for assessment if the fever persists). The selection of appropriate antibiotics is something intended to be covered by local IMCI adaptations (WHO and UNICEF 1999). However, to date there is simply very little evidence available on fever aetiology and drug susceptibility in resource-limited settings. Recent electronic algorithms with an ‘IMCI backbone’ (Keitel et al. 2017, 4) have begun to offer more specific instructions to reduce antibiotic use, and to incorporate not only clinical signs but also biomarker point-of-care tests such as C-reactive protein (see Keitel and D’Acremont 2018). While such tests can indicate when an antibiotic might be needed based on defined biomarker cut-off points, defining suitable cut-offs is a challenge; moreover, these tests do not indicate which antibiotic should be used. Thus, while the benign-looking green of the nonmalarial fever category remains, one might argue that this is merely a convention carried over from the older system and is befitting of a more alarming yellow or red, especially given rising concerns over global AMR and the threat of an impending ‘antibiotic apocalypse’ (McKie 2017).
Opening up ‘fever’, closing down medicines
As collaborators in a multicountry fever aetiology study, we have observed a growing push to disentangle the category of fever from malaria, and to establish it as a discrete object of research, policy, and intervention. It has been suggested, for instance, that like diarrhoea and lower-respiratory-tract infections, febrile illness could be conceived of as a syndromic ‘envelope’ that ‘contains’ numerous pathogens but also represent an organising category for which global burden of disease estimates could be produced through rigorous aetiology research (Crump and Kirk 2015; Maze et al. 2018). At the clinical level, it is hoped that aetiology data and diagnostic innovations will enable the refinement of clinical algorithms such as IMCI, with diagnostics, in particular, being heavily invested in through FIND (2015), Unitaid (2018), and public-private partnerships. Considerable diagnostics research is currently underway, with the goal of an RDT that can accurately distinguish bacterial from nonbacterial causes of acute fever and thereby drive down rates of antimicrobial prescription (Dittrich et al. 2016). It is possible that future iterations of the IMCI algorithm could include a two-step diagnostic process for febrile children: first the malaria RDT and then, in the case of a negative result, a second RDT for bacterial infection. In short, considerable funding and attention is being devoted to opening up the category of ‘fever’ and inserting tools for its clinical management into the infrastructure of the clinic.
From an anthropological perspective, we might ask: will aetiology studies, diagnostics, and algorithmic refinement finally ‘complete’ the fever category, disrupt the malaria silo, and secure more comprehensive care for fever? Or might they create further grooves that close down possibilities as much they open them, obscure as much as they reveal, and create new residual ‘other’ categories and possibilities (or not) for medicines in the process? Looking back at the history of IMCI, we can see a shift in which the algorithm, first designed as a low-tech tool to empirically treat the most common causes of childhood mortality, is fast becoming a decidedly high-tech tool mediated by diagnostics. Yet, as Bowker and Star (1999) importantly observe, new classification work is always constrained by what is already in place, in this case the algorithm’s original shape, structure, and intentions, as well as the entire system that has been built around it over years and decades. Whilst these new diagnostics entail significant classificatory knock-on effects as they are being inserted, resulting in new visibilities such as ‘nonmalarial fever’, the playing field upon which they are being layered remains one where medicines remain central to care. Arguably, the sudden spike in antibiotic use reflects not simply the lack of current guidance in the algorithm regarding antibiotics, but rather a deep systemic reliance upon medicines that has been displaced onto the next class of antimicrobial.
What is changing, however, and seemingly only further obscuring the lack of alternatives for care, is the way that diagnostics are being appealed to as solutions to health challenges in LMICs (Engel 2017; Street 2018). The phrase ‘access to diagnostics’ (see for example FIND 2015, 12), both for patients and health providers, currently lies at the centre of the rhetorical push for diagnostics. The assumption underlying this curiously paradoxical phrase is that access to diagnostics is the same as access to care, in much the same way that the blanket, presumptive coverage of medicines was framed beforehand. Yet it is interesting to note that, at least in the recent history of global health, diagnostics are more about ruling out disease and the need for medicines than they are about ruling them in, which is especially evident in current debates and discourses around febrile illness. To say ‘access to diagnostics’ is to say access to a gatekeeper of medicines; with the insertion of diagnostics into the algorithm, front-line care is being subtly reorganised to be less about access to medicines and more about who qualifies for these precious, threatened commodities and who does not. The category of ‘fever’, which was previously a gateway to medicines, is now a gateway to a gatekeeper in which the empirical, experiential, and relational (which previously held much sway in the negotiation of diagnosis) are being short-circuited in favour of the test. Thus, in the same moment that the fever script is becoming more open, refined, and sophisticated, leading to the appearance of improvements in care, this is the result of a perpetual game of Whack-a-Mole[note 2] in which medicine options are being gradually shut down.
Why me? Why now?
Although ‘fever’ is the only IMCI primary clinical sign for which an RDT has yet been written into the script, the drive towards novel diagnostics and the imperative to ‘rationalise’ the use of medicines cross-cuts the spectrum of the IMCI’s classifications. It is, indeed, currently reshaping the very way that health care in LMICs is being framed and addressed. With the WHO’s (2015) Global Action Plan and numerous national action plans on AMR currently being implemented, and new revisions to the IMCI guidelines currently being drafted, we are likely to see such imperatives increasingly reflected within clinical algorithms in the coming years. At the same time, the flattening of experience and acumen is being accompanied by greater scrutiny of the ‘irrational’ and ‘inappropriate’ practices of clinicians and patients (O’Neill 2016).
To shed light upon the persisting and novel inequalities that are being (re)produced by the shifting classificatory blueprints for global health, it is important not only to critically ‘read the classifications themselves’ (Bowker and Starr 1999, 55) – as we have attempted to do here – but to also understand what these processes look like from the other direction, that is, from the perspective of the clinicians, patients, and other actors who are most affected by them on the ground. For amidst the increasing orientation towards the rationalising and rationing of medicines, people are still grappling with the same age-old questions that anthropologists like Evans-Pritchard (1937) were studying in the early to mid-twentieth century: ‘Why me (or my child)? Why now?’ Despite the rhetoric of certainty surrounding new diagnostics, these are questions that diagnostics are arguably simply ill-equipped to answer. Conducting qualitative research in the context of a clinical trial in northern Thailand, Haenssgen and colleagues (2018) recently found that the C-reactive protein test, which was intended to reduce antibiotic prescription, was viewed favourably by both clinicians and patients. However, when negative results conflicted with clinical judgement, results could be bypassed; moreover, patients often still expected a prescription, an expectation informed by the deeply rooted conception that pharmaceuticals represent ‘good care’. These findings, similar to those of ethnographic studies of malaria RDTs (see for example Beisel et al. 2016; Chandler et al. 2012), are perhaps unsurprising in light of our reading, and support the notion that uncertainty will not simply dissipate just because more diagnostics are added to the system. To the contrary, uncertainty is arguably an inevitable by-product of this increasingly high-tech form of pharmaceuticalised care being introduced into LMICs.
One noteworthy feature of the emerging era of AMR is how domains of illness and experience previously considered ordinary, mundane, and residual by the standards of global health are suddenly being cast into the spotlight. As we have shown in our classificatory reading here, from systematic attempts to get people onto malaria treatment (on the basis of being febrile), there is now a push to get people off medicines when they have ‘fever’. This inversion of priorities in turn opens up space for ethnographic enquiry into how people classify and engage ‘ordinary’ illnesses in the era of AMR. How does something come to be classified as a ‘fever’? How, as Canguilhem (1966) would ask, does a fever come to be classified as ‘normal’ or ‘pathological’? When, as Kamat (2006) has enquired more recently, does a fever come to be classified as ‘ordinary’ and when is it more severe, requiring antimicrobial treatment? How is the shifting script of IMCI (re)shaping everyday experiences of illness, health care seeking, and health care delivery in LMICs, and how does it intertwine with other factors that affect people’s vulnerability, including family and social networks, social status, gender, and local politics? At the same time, amidst the increasing delegitimisation of experience and acumen, how is the script borrowed and navigated by providers, patients, and other actors in order to secure certainty and ‘good care’? Such questions cross-cut a range of anthropological and social science approaches, including critical global health, Bowker and Star’s (1999) attention to formal classification systems, and older illness classification studies in the tradition of ethnomedicine. But taken together, they highlight the importance of attending to ‘classification work’ in the governance of health and how this work plays out in local material and moral worlds.
Conclusion
In this article, we have drawn upon the analysis of Bowker and Star (1999) to propose ‘classification work’ as a lens through which to view the shifting blueprints for global health in the era of AMR. Using the IMCI algorithm as our example and, more specifically, its treatment of the fever category (both figurative and literal), we have demonstrated the influence of formal classification systems in the (re)making of global health. Our aim in providing a critical reading has been twofold: firstly, to demonstrate the continued and perhaps reinvigorated systemic reliance upon medicines to provide care; and secondly to show that upon this algorithmic base is being layered an increasingly high-tech but ‘empty’ form of pharmaceuticalised care in which blame for continued ‘overuse’ is displaced onto the ‘irrational’ behaviours of end-users. Despite the undoubted importance of addressing the neglect of illnesses that were previously treated as malaria, this emptiness is reflected in how the opening-up and refinement of ‘fever’ belies a corresponding closing down of medicine options.
Our attention to the pervasive yet sunken, material yet malleable classificatory blueprints upon which global health is built is one angle from which to approach the ‘access-excess’ debate (Laxminarayan et al. 2013). Just as antimicrobial use practices are increasingly being branded as ‘irrational’ and ‘irresponsible’, we argue that not only is the imperative to secure access to medicines slipping ever further from view but also that much of the apparent ‘excess’ has been conditioned for years by formal classification work initiated in the global North. At the same time, debates around ‘access’ and ‘excess’ tend to remain focused on medicines. While it is important to ensure that access to medicines remains on the global health agenda, in order to keep vulnerable people from slipping from view it is important also to ensure that discussions around access do not stop short of considering care beyond its pharmaceuticalised form in LMICs (Biehl 2007; Biehl and Petryna 2013; Nguyen 2010). This is becoming increasingly hard to consider, for as we have shown here, the centrality of medicines has become embedded in infrastructure, discourse, and even the words that we used to describe illness. Research that aims to configure stewardship of antimicrobials in the era of concern about AMR must attend to whether patients are categorised as targets for ‘case management’ or for ‘care’.
Acknowledgements
Research for this article was supported by the Department for International Development (FIEBRE Project PO7856) and the Antimicrobial Resistance Cross Council Initiative through the Economic and Social Research Council (AMIS Project ES/P008100/1). We would also like to thank our colleagues from the FIEBRE and AMIS projects and the ACT Consortium for their insights, particularly Heidi Hopkins, Eleanor MacPherson, Coll Hutchison, Laurie Denyer Willis, Sarah Staedke, and Christopher Whitty. We finally thank Andrew Gomez for assistance with image design for the figures.
About the authors
Justin Dixon is Research Fellow in Medical Anthropology at the London School of Hygiene and Tropical Medicine and a member of the Anthropology of Antimicrobial Resistance research group. He currently works on a multidisciplinary study called FIEBRE in Africa and Southeast Asia, exploring what ‘care’ means in increasingly restrictive prescribing environments. His PhD work looked at bioethics and configurations of the human subject in tuberculosis vaccine trials in South Africa. Justin.Dixon@lshtm.ac.uk
Clare Chandler is Professor in Medical Anthropology at the London School of Hygiene and Tropical Medicine where she is Director of the Antimicrobial Resistance Centre and leads the Anthropology of Antimicrobial Resistance research group. Her current research focuses on the work antimicrobials do in health care and in societies more widely. This extends her previous research on medicines and diagnostics use in east Africa. Clare.Chandler@lshtm.ac.uk
References
Agamben, Giorgio. 1995. Homo Sacer: Sovereign Power and Bare Life. Stanford, CA: Stanford University Press.
Allen, Tim, and Melissa Parker. 2011. ‘The “Other Diseases” of the Millennium Development Goals: Rhetoric and Reality of Free Drug Distribution to Cure the Poor’s Parasites’. Third World Quarterly 32 (1): 91–117. https://doi.org/10.1080/01436597.2011.543816.
Armstrong, D. 2002. ‘Clinical Autonomy, Individual and Collective: The Problem of Changing Doctors’ Behaviour’. Social Science & Medicine 55: 1771–77. https://doi.org/10.1016/S0277-9536(01)00309-4.
Baer, Hans A., Merrill Singer, and Ida Susser. 1997. Medical Anthropology and the World System. Westport, CT: Bergin & Garvey.
Banco, Leonard, and Daniel Veltri. 1984. ‘Ability of Mothers to Subjectively Assess the Presence of Fever in Their Children’. American Journal of Diseases of Childhood 138: 976–78.
Batwala, Vincent, Pascal Magnussen, and Fred Nuwaha. 2011. ‘Antibiotic Use among Patients with Febrile Illness in a Low Malaria Endemicity Setting in Uganda’. Malaria Journal 10: 1–8. https://doi.org/10.1186/1475-2875-10-377.
Beisel, Uli, Rene Umlauf, Eleanor Hutchinson, and Clare Chandler. 2016. ‘The Complexities of Simple Technologies: Re-imagining the Role of Rapid Diagnostic Tests in Malaria Control Efforts’. Malaria Journal 15 (1): 64. https://doi.org/10.1186/s12936-016-1083-2.
Biehl, João G. 2007. ‘Pharmaceuticalization: AIDS Treatment and Global Health Politics’. Anthropological Quarterly 80 (4): 1083–1126. https://doi.org/10.1353/anq.2007.0056.
Biehl, Joao G., and Adriana Petryna, eds. 2013. When People Come First: Critical Studies in Global Health. Princeton, NJ: Princeton University Press.
Bowker, Geoffrey C., and Susan L. Star. 1999. Sorting Things Out: Classification and Its Consequences. Cambridge, MA: MIT Press.
Brown, Nik, and Sarah Nettleton. 2017. ‘“There Is Worse to Come”: The Biopolitics of Traumatism in Antimicrobial Resistance (AMR)’. The Sociological Review 65 (3): 493–508. https://doi.org/10.1111/1467-954X.12446.
Bruun Jensen, Casper, and Brit R. Winthereik. 2013. Monitoring Movements in Development Aid: Recursive Partnerships and Infrastructures. Cambridge, MA: MIT Press.
Burchett, Helen E. D., Baptiste Leurent, Frank Baiden, Kimberly Baltzell, Anders Björkman, Katia Bruxvoort, Siân Clarke, et al. 2017. ‘Improving Prescribing Practices with Rapid Diagnostic Tests (RDTs): Synthesis of 10 Studies to Explore Reasons for Variation in Malaria RDT Uptake and Adherence’. BMJ Open 7: e012973. https://doi.org/10.1136/bmjopen-2016-012973.
Canguilhem, Georges. (1966) 1991. The Normal and the Pathological. New York: Zone Books.
Chandler, Clare I. R. 2019. ‘Current Accounts of Antimicrobial Resistance: Stabilisation, Individualisation and Antibiotics as Infrastructure’. Palgrave Communications 5 (53). https://doi.org/10.1057/s41599-019-0263-4.
Chandler, Clare I. R., and Uli Beisel. 2017. ‘The Anthropology of Malaria: Locating the Social’. Medical Anthropology 36 (5): 411–21.https://doi.org/10.1080/01459740.2017.1306858.
Chandler, Clare I. R., Eleanor Hutchinson, and Coll Hutchison. 2016. Addressing Antimicrobials through Social Theory: An Anthropolgically Oriented Report. London: London School of Hygiene and Tropical Medicine.http://app.lshtm.ac.uk/files/2016/11/LSHTMAnthroAMR-2016.pdf.
Chandler, Clare I. R., Caroline Jones, Gloria Boniface, Kaseem Juma, Hugh Reyburn, and Christopher J. M. Whitty. 2008. ‘Guidelines and Mindlines: Why Do Clinical Staff Over-diagnose Malaria in Tanzania? A Qualitative Study’. Malaria Journal 7: 53. https://doi.org/10.1186/1475-2875-7-53.
Chandler, Clare I. R., Lindsey Mangham, Abanda N. Njei, Olivia Achonduh, Wilfred F. Mbacham, and Virginia Wiseman. 2012. ‘“As a Clinician, You Are Not Managing Lab Results, You Are Managing the Patient”: How the Enactment of Malaria at Health Facilities in Cameroon Compares with New WHO Guidelines for the Use of Malaria Tests’. Social Science & Medicine 74 (10): 1528–35. https://doi.org/10.1016/j.socscimed.2012.01.025
Chandler, Clare. I. R., Emily L. Webb, Catherine Maiteki-Sebuguzi, Susan Nayiga, Christine Nabirye, Deborah D. DiLiberto, Emmanuel Ssemmondo, Grant Dorsey, Moses R. Kamya, and Sarah G. Staedke. 2017. ‘The Impact of an Intervention to Introduce Malaria Rapid Diagnostic Tests on Fever Case Management in a High Transmission Setting in Uganda: A Mixed-Methods Cluster-Randomized Trial (PRIME)’. PLoS One 12 (3): e0170998. https://doi.org/10.1371/journal.pone.0170998.
Chandramohan, Daniel, Shabbar Jaffar, and Brian Greenwood. 2002. ‘Use of Clinical Algorithms for Diagnosing Malaria’. Tropical Medicine and International Health 7 (1): 45–52. https:/doi.org/10.1046/j.1365-3156.2002.00827.
Crump, John, and Martyn Kirk. 2015. ‘Estimating the Burden of Febrile Illnesses’. PLoS Neglected Tropical Diseases 9 (12): e0004040. https://doi.org/10.1371/journal.pntd.0004040.
Dittrich, Sabine, Sabine Dittrich, Birkneh Tilahun Tadesse, Francis Moussy, Arlene Chua, Anna Zorzet, Thomas Tängdén, et al. 2016. ‘Target Product Profile for a Diagnostic Assay to Differentiate between Bacterial and Non-Bacterial Infections and Reduce Antimicrobial Overuse in Resource-Limited Settings: An Expert Consensus’. PLoS ONE 11 (8): e0161721. https://doi.org/10.1371/journal.pone.0161721.
Dixon, Justin, and Michèle Tameris. 2019. ‘Between Representing and Intervening: Diagnosing Childhood Tuberculosis during a Vaccine Trial in South Africa’. In Understanding Tuberculosis and Its Control: Anthropological and Ethnographic Approaches, edited by Helen Macdonald and Ian Harper, 241–58. London and New York: Routledge.
Durkheim, Emile, and Marcel Mauss. (1902) 1963. Primitive Classification. Translated by Rodney Needham. Chicago: University of Chicago Press.
Engel, Nora, Vijayashree Yellappa, Nitika Pant Pai, and Madhukar Pai. 2017. ‘Diagnosing at Point of Care: Coordination Work and Frictions’. Science & Technology Studies 30 (3): 54–72.
Evans-Pritchard, Edward E. 1937. Witchcraft, Oracles and Magic among the Azande. Oxford: Oxford University Press.
Feierman, Steven. 2011. ‘When Physicians Meet: Local Medical Knowledge and Global Public Goods’. In Evidence, Ethos and Experiment: The Anthropology and History of Medical Research in Africa, edited by Paul Wenzel Geissler, 171–96. New York: Berghahn Books.
Ferguson, James. 1990. The Antipolitics Machine: Development, Depoliticization, and Bureaucratic Power in Lesotho. Minneapolis: University of Minnesota Press.
Foster, George. M. 1976. ‘Disease Etiologies in Non‐Western Medical Systems’. American Anthropologist 78 (4): 773–82.
FIND (Foundation for Innovative New Diagnostics). 2015. Turning Complex Diagnostic Challenges: Strategy 2015–2020. https://www.finddx.org/wp-content/uploads/2016/01/FIND_Strategy.pdf.
Good, Byron J. 1977. ‘The Heart of What’s the Matter: The Semantics of Illness in Iran’. Culture, Medicine and Society 1: 25–58.
Haenssgen, Marco, Nutcha Charoenboon, Thomas Althaus, Rachel C. Greer, Daranee Intralawan, and Yoel Lubell. 2018. ‘The Social Role of C-reactive Protein Point-of-Care Testing to Guide Antibiotic Prescription in Northern Thailand’. Social Science & Medicine 202: 1–12. https://doi.org/10.1016/j.socscime.d.2018.02.018.
Haenssgen, Marco, Nutcha Charoenboon, and Yuzana Khine Zaw. 2018. ‘It Is Time to Give Social Research a Voice to Tackle Antimicrobial Resistance?’ Journal of Antimicrobial Chemotherapy 73 (4): 1112–13. https://doi.org/10.1093/jac/dkx533.
Hausmann-Muela, Susanna, Joan Muela Ribera, and Marcel Tanner. 1998. ‘Fake Malaria and Hidden Parasites: The Ambiguity of Malaria’. Anthropology and Medicine 5 (1): 43–61.
Helman, Cecil G. 2007. Culture, Health and Illness. 5th ed. London: Hodder Arnold.
Herrick, Claire. 2017. ‘The (Non)Charisma of Noncommunicable Diseases’. Social Theory and Health 15 (1): 99–116. https://doi.org/10.1057/s41285-016-0021-2.
Hopkins, Heidi, Caroline Asiimwe, and David Bell. 2009. ‘Access to Antimalarial Therapy: Accurate Diagnosis Is Essential to Achieving Long Term Goals’. BMJ 339: b2606. https://doi.org/10.1136/bmj.c2063.
Hopkins, Heidi, Katia J. Bruxvoort, Matthew E. Cairns, Clare I. R. Chandler, Baptiste Leurent, Evelyn K Ansah, Frank Baiden, et al. 2017. ‘Impact of Introduction of Rapid Diagnostic Tests for Malaria on Antibiotic Prescribing: Analysis of Observational and Randomised Studies in Public and Private Healthcare Settings’. BMJ 356: j1054. https://doi.org/10.1136/bmj.j1054.
Human, Oliver. 2011. ‘The Rings around Jonathon’s Eyes: HIV/AIDS Medicine at the Margins of Administration’. Medical Anthropology 30 (2): 222–39.
Hutchinson, Eleanor. 2016. ‘Part 2: Applying Social Theory to Antimicrobial Resistance Policy’. In Addressing Antimicrobials through Social Theory: An Anthropolgically Oriented Report, by Clare Chandler, Eleanor Hutchison, and Coll Hutchison. London: London School of Hygiene and Tropical Medicine. http://app.lshtm.ac.uk/files/2016/11/LSHTMAnthroAMR-2016.pdf.
Hutchinson, Eleanor, Coll Hutchison, Sham Lal, Kara Hanson, Miriam Kayendeke, Christine Nabirye, P. Magnussen, et al. 2017. ‘Introducing Rapid Tests for Malaria into the Retail Sector: What are the Unintended Consequences?’ BMJ Global Health 2(1): e000067. https://doi.org/10.1136/bmjgh-2016-000067.
Hutchison, Coll, Gwen Knight, Richard Stabler, and Clare Chandler. 2018. ‘The Modern Era Must End: Antibiotic Resistance Helps Us Rethink Medicine and Farming’. BMJ Opinion (blog). 11 July. https://blogs.bmj.com/bmj/2018/07/11/the-modern-era-must-end-antibiotic-resistance-helps-us-rethink-medicine-and-farming/.
Hutchinson, Eleanor, Hugh Reyburn, Eleanor Hamlyn, Katie Long, Judith Meta, Hilda Mbakilwa, and Clare I. R. Chandler. 2015. ‘Bringing the State into the Clinic? Incorporating the Rapid Diagnostic Test for Malaria into Routine Practice in Tanzanian Primary Healthcare Facilities’. Global Public Health. 12 (9): 1077–91. https://doi.org/10.1080/17441692.2015.1091025.
Jacobs, Marian, and Michael Merson. 2018. ‘Introductory Commentary: A Strategic Review of Options for Building on Lessons Learnt from IMCI and iCCM’. BMJ 362. https://doi.org/10.1136/bmj.k3013.
Johansson, Emily W., Katarina E. Selling, Humphreys Nsona, Bonnie Mappin, Peter W. Gething, Max Petzold, Stefan Swartling Peterson, and Helena Hildenwall. 2016. ‘Integrated Paediatric Fever Management and Antibiotic Over-Treatment in Malawi Health Facilities: Data Mining a National Facility Census’. Malaria Journal 15 (1): 1–12. https://doi.org/10.1186/s12936-016-1439-7.
Kamat, Vinay R. 2006. ‘“I Thought It Was Only Ordinary Fever!” Cultural Knowledge and the Micropolitics of Therapy Seeking for Childhood Febrile Illness in Tanzania’. Social Science & Medicine 62 (12): 2945–59. https://doi.org/10.1016/j.socscimed.2005.11.042.
Kamat, Vinay R. 2008. ‘Dying under the Bird’s Shadow: Narrative Representations of Degedege and Child Survival among the Zaramo of Tanzania’. Medical Anthropology Quarterly 22 (1): 67–93. https://www.doi.org/0.1111/j.1548-1387.2008.00004.x.
Keitel, Kristina, and Valérie D’Acremont. 2018. ‘Electronic Clinical Decision Algorithms for the Integrated Primary Care Management of Febrile Children in Low-Resource Settings: Review of Existing Tools’. Clinical Microbiology and Infection 24 (8): 845–55. https://doi.org/10.1016/j.cmi.2018.04.014.
Keitel, Kristina, Frank Kagoro, Josephine Samaka, John Masimba, Zamzam Said, Hosiana Temba, Willy Sangu Clotilde Rambaud-Althaus, et al. 2017. ‘A Novel Electronic Algorithm Using Host Biomarker Point-of-Care-Tests for the Management of Febrile Illnesses in Tanzanian Children (e-POCT): A Randomized, Controlled, Non-Inferiority Trial’. PLoS Medicine 14 (10): e1002411. https://doi.org/10.1371/journal. pmed.1002411.
Lambert, Helen. 2006. ‘Accounting for EBM: Notions of Evidence in Medicine’. Social Science & Medicine 62: 2633–45. https://www.doi.org/10.1016/j.socscimed.2005.11.023.
Langwick, Stacey A. 2007. ‘Devils, Parasites and Fierce Needles: Healing and the Politics of Translation in Southern Tanzania’. Science, Technology and Human Values 32: 88–117.
Latour, Bruno. 1979. Laboratory Life: The Construction of Scientific Facts. Princeton, NJ: Princeton University Press.
Laxminarayan, Ramanan, Adriano Duse, Chand Wattal, Anita K. M. Zaidi, Heiman F. L. Wertheim, Nithima Sumpradit, Erika Vlieghe, et al. 2013. ‘Antibiotic Resistance: The Need for Global Solutions’. Lancet Infectious Diseases. 13 (12): 1057–98. https://doi.org/10.1016/S1473-3099(13)70318-9.
Livingstone, Julie. 2012. Improvising Medicine: An African Oncology Ward in an Emerging Cancer Epidemic. Durham, NC: Duke University Press.
Marks, Harry. 1997. The Progress of Experiment: Science and Therapeutic Reform in the United States, 1900–1990. Cambridge: Cambridge University Press
Maze, Michael J., Quique Bassat, Nicholas A. Feasey, Inácio Mandomando, Patrick Musicha, and John A. Crump. 2018. ‘The Epidemiology of Febrile Illness in Sub-Saharan Africa: Implications for Diagnosis and Management’. Clinical Microbiology and Infection 24 (8): 808–814. https://doi.org/10.1016/j.cmi.2018.02.011.
McKie, Robin. 2017. ‘“Antibiotic Apocalypse” Doctors Sound Alarm over Drug Resistance’. Guardian, 8 October. https://www.theguardian.com/society/2017/oct/08/world-faces-antibiotic-apocalypse-says-chief-medical-officer.
Mills, Anne. 1983. ‘Vertical vs. Horizontal Health Programmes in Africa: Idealism, Pragmatism, Resources and Efficiency’. Social Science & Medicine17(24): 1971–81.
Nguyen, Vinh-Kim. 2010. The Republic of Therapy: Triage and Sovereignty in West Africa’s Time of AIDS. Durham, NC: Duke University Press.
Nichter, Mark. 1996. ‘Health Social Science Research on the Study of Diarrhoeal Disease: A Focus on Dysentery’. In Anthropology and International Health: Asian Case Studies, by Mark Nichter and Mimi Nichter, 111–29. London: Routledge.
Nichter, Mark. 2008. Global Health: Why Cultural Perceptions, Social Representations, and Biopolitics Matter. Tuscon: University of Arizona Press.
Nichter, Mark, and Mimi Nichter. 1996. Anthropology and International Health: Asian Case Studies. London: Routledge.
Ogren, J. 1990. ‘The Inaccuracy of Axillary Measures Using an Electric Thermometer’. American Journal of Diseases of Childhood 144: 109–111. https://doi.org/10.1001/archpedi.1990.02150250121048.
O’Neill, Jim. 2016. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. The Review on Antimicrobial Resistance. Chaired by Jim O’Neill. London: The Review on Antimicrobial Resistance. https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf.
Perkins, B. A., J. Zucker, B. Schwartz, and J. Otieno. 1997. ‘Evaluation of an Algorithm for Integrated Management of Childhood Illness in an Area of Kenya with High Malaria Transmission’. Bulletin of the World Health Organisation 75: 33–42.
Pfeiffer, James, and Mark Nichter (for the Critical Anthropology of Global Health Special Interest Group). 2008. ‘What Can Critical Medical Anthropology Contribute to Global Health? A Health Systems Perspective’. Medical Anthropology Quarterly 22 (4): 410–15. https://doi.org/10.1111/j.1548-1387.2008.00041.x.
Prince, Ruth J., and Rebecca Marsland, eds. 2014. Making and Unmaking Public Health in Africa: Ethnographic and Historical Perspectives. Athens: Ohio University Press.
Reyburn, Hugh, Hilda Mbakilwa, Rose Mwangi, Ombeni Mwerinde, Raimos Olomi, Chris Drakeley, and Christopher J. M. Whitty. 2007. ‘Rapid Diagnostic Tests Compared with Malaria Microscopy for Guiding Outpatient Treatment of Febrile Illness in Tanzania: Randomised Trial’. BMJ 334: 403. https://doi.org/10.1136/bmj.39073.496829.AE.
Reyburn, Hugh, Redepmta Mbatia, Chris Drakeley, Ilona Carneiro, Emmanuel Mwakasungula, Ombeni Mwerinde, Kapalala Saganda, et al. 2004. ‘Overdiagnosis of Malaria in Patients with Severe Febrile Illness in Tanzania: A Prospective Study’. BMJ 329: 1212. https://doi.org/10.1136/bmj.38251.658229.55.
Ribera, Joan Muela, and Susannah Haussmann-Muela. 2011. ‘The Straw That Breaks the Camel’s Back: Redirecting Health-Seeking Behavior Studies on Malaria and Vulnerability’. Medical Anthropology Quarterly 25 (1): 103–121.
Rowe, A., K. G. Hirnschall, T. Lambrechts, and J. Bryce. 1999. ‘Linking the Integrated Management of Childhood Illness (IMCI) and Health Information System (HIS) Classifications: Issues and Options’. Bulletin of the World Health Organisation 77 (12): 988–95.
Star, Susan. Leigh. 1999. ‘The Ethnography of Infrastructure’. American Behavioural Scientist 43 (3): 377–91. https://doi.org/10.1177/00027649921955326.
Street, Alice. 2014. ‘Rethinking Infrastructures for Global Health: A View from West Africa and Papua New Guinea’. Somatosphere, 12 December. http://somatosphere.net/2014/12/rethinking-infrastructures.html.
Street, Alice. 2018. ‘The Testing Revolution: Investigating Diagnostic Devices in Global Health’. Somatosphere, 9 April. http://somatosphere.net/2018/04/testing-revolution.html.
Thgawa, Tatsou. 1985. ‘Body Temperature Measurement’. Clinical Physics and Phyiological Measures 6: 83–10.
Timmermans, Stefan, and Marc Berg. 2003. The Gold Standard: The Challenge of Evidence-Based Medicine and Standardization in Health Care. Philadelphia, PA: Temple University Press.
Umlauf, René, and Sung-Joon Park. 2017. ‘Stock-outs! Improvisations and Processes of Infrastructuring in Uganda’s HIV/Aids and Malaria Programmes’. Global Public Health 13: 325–38. https://doi.org/10.1080/17441692.2017.1414287.
Unitaid. 2018. Technology Landscape: Fever Technology Diagnostic Landscape. 1st ed. https://unitaid.eu/assets/Fever_diagnostic_technology_and_market_landscape.pdf.
Walsh, Fergus. 2014. ‘Antibiotic Resistance: Cameron Warns of Medical “Dark Ages”’. BBC News, 2 July. http://www.bbc.co.uk/news/health-28098838.
Weber, M. W., E. K. Mulholland, S. Jaffar, H. Troedsson, S. Gove, and B. M. Greenwood. 1997. ‘Evaluation of an Algorithm for the Integrated Management of Childhood Illness in an Area with Seasonal Malaria in the Gambia’. Bulletin of the World Health Organisation 75 (supplement 1): 25–32.
WHO. 1993. The Management of Fever in Young Children with Acute Respiratory Infections in Developing Countries. Geneva: World Health Organisation. http://apps.who.int/iris/handle/10665/58266.
WHO. 2010. Guidelines for the Treatment of Malaria. 2nd ed. Geneva: World Health Organisation. http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf.
WHO. 2015. Global Action Plan on Antimicrobial Resistance. Geneva: World Health Organisation. https://www.who.int/antimicrobial-resistance/global-action-plan/en/.
WHO. 2017. Integrated Management of Childhood Illness Global Survey Report. Geneva: World Health Organisation. http://www.who.int/maternal_child_adolescent/documents/imci-global-survey-report/en/.
WHO and UNICEF. 1999. IMCI Information. Geneva: World Health Organisation. https://www.who.int/maternal_child_adolescent/documents/chs_cah_98_1a/en/.
WHO and UNICEF. 2002. IMCI Adaptation Guide Part 1: The Adaptation Process and Procedures for Adapting the Charts and Modules. Geneva: World Health Organisation. http://www.who.int/maternal_child_adolescent/documents/pdfs/imci_adaptation_guide_1a.pdf.
WHO and UNICEF. 2008. Integrated Management of Childhood Illnesses (IMCI) Chart Booklet. 2nd ed. https://www.scribd.com/document/8585609/IMCI-Chart-Booklet-2008-edition-Integrated-Management-of-Childhood-Illness-World-Health-Organization-WHO-United-Nations-International-Children-s-Eme.
WHO and UNICEF. 2014. Integrated Management of Childhood Illnesses (IMCI) Chart Booklet. 3rd ed. http://www.who.int/maternal_child_adolescent/documents/IMCI_chartbooklet/en/.
Williams, H. A., and C. O. H. Jones. 2004. ‘A Critical Review of Behavioural Issues Related to Malaria Control in Sub-Saharan Africa: What Contributions Have Social Scientists Made?’ Social Science & Medicine 59: 501–23.
Wolfheim, Cathy. 1998. ‘From Disease Control to Child Health and Development’. World Health Forum 19: 174–81.
Endnotes
1 Back
The Integrated Management of Adolescent and Adult Illness (IMAI) guideline was designed slightly later in the early 2000s. As it contains many similarities to IMCI, our argument might usefully be applied to the IMAI too, although it is beyond the scope of this article.
2 Back
This is the name of an arcade game in which small figures of moles pop up randomly in a field of holes, and the players attempt to hit them all; as one mole is hit, another one appears.