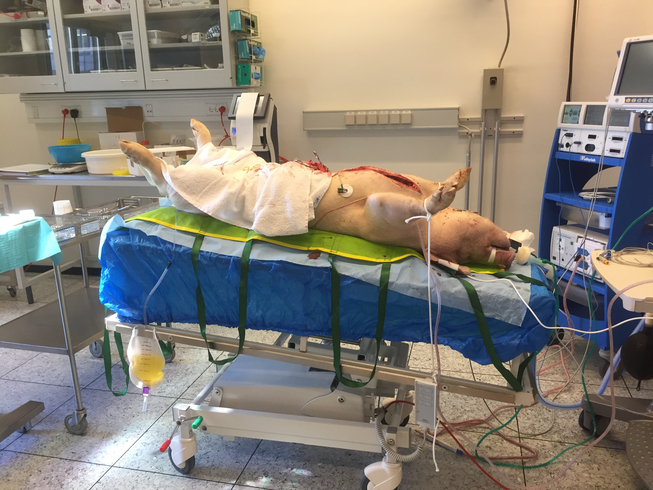
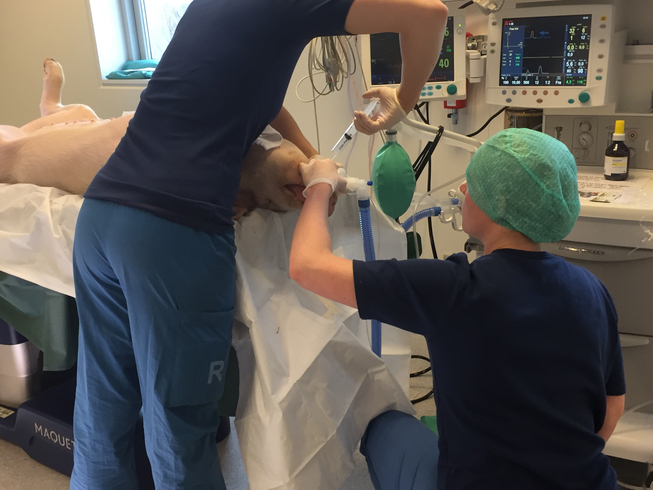
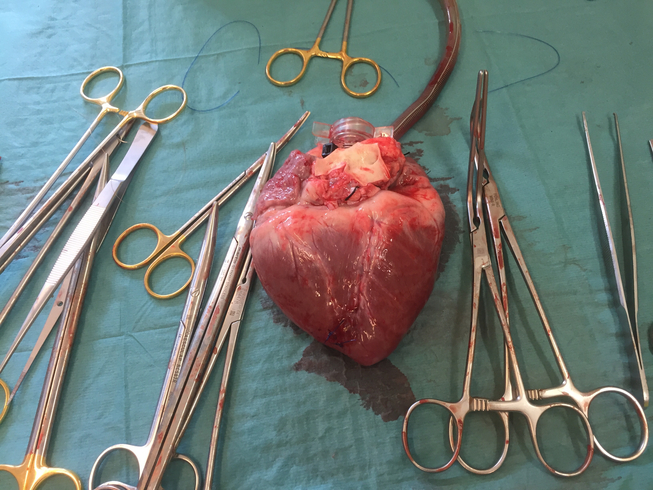
Pigs and pig organs are frequently used prior to human trials in experimental transplant research into how to optimise human transplantation. But what exactly happens when transplant professionals perform experimental research on pigs? Similarly, what happens when a pig is on the surgical table? Based on ethnographic fieldwork in Danish transplant research laboratories, we investigate how pig experiments facilitate ‘collaborative intimacies’ among medical professionals. Collaborative intimacies are used here as an empirical and theoretical framework for conceptualising and re-imagining the social relationships between species and the medical disciplines that emerge in laboratory work. Collaborative intimacies in the lab provide medical training and facilitate moral reflection and social networking among transplant professionals. As such, we argue that research utilising animal models is not only about technological progress and ethical dilemmas; rather, collaborative intimacies make us understand how intimate relations among medical professionals in translational research unfold and how such relations matter for professional and technological futures.
Collaborative intimacies
How research pigs in Danish organ transplantation facilitate medical training, moral reflection, and social networking
—
Abstract
Introduction
Worldwide, the field of organ transplantation is characterised by the endless quest for exchangeable human organs to save and improve the lives of sick patients. The difficulties associated with trying to increase the number of potential donors have resulted in a multitude of research investments worldwide into technological solutions to optimise the available number of organs. In various experimental studies devoted to developing and improving knowledge and practices of transplantation, pigs are used as models for human donors and recipients. Danish research experiments in this field constitute the ethnographic foundation of this article.
Despite the Danes’ positive attitude towards transplantation (Nordfalk et al. 2016), Denmark faces an ongoing need for exchangeable and functional human organs. Currently, Denmark only procures organs from brain-dead donors, but the Danish Health Authority plans on implementing donation after circulatory death (DCD) in 2021, aiming to expand the number of potential donors. In DCD donation, intensive care patients on ventilators with no hopes of survival are considered potential donors. The DCD donor patient is extubated, after which the heart stops. After a ‘no-touch period’ that across Europe varies between five and 20 minutes (Lomero et al. 2019), the patient is declared dead and the organs are removed. The definition of this death criterion, its implementation, and the clinical practices and ethical dilemmas it generates have caused international debate (Cooper 2018; Dalle Ave et al. 2020; Jericho 2019). Unlike organs from brain-dead donors (who are on ventilators until organ removal), organs from DCD donors might suffer from the temporary lack of oxygen and perfusion that the ‘no-touch period’ entails. In other words, if DCD organs are not handled utilising the best possible medical technology and surgical skills, transplantation procedures may fail due to poor organ function. Consequently, Danish heart and lung surgeons have initiated a number of research experiments on pigs to train for a future where DCD organs can be procured and preserved using the newest technological options. In the Danish transplant community, these pigs are often called ‘transplant pigs’, a term we have adopted for this article. The transplant pigs must not be confused with the genetically edited pigs created by international xenotransplantation scientists hoping to one day transplant pig organs into human patients (an ‘experimental world’ that has already caught the interest of several prominent scholars of the social sciences [Rémy 2009; Sharp 2013]); in our material, transplant pigs are models, not donors, and pig organs are used for research only.
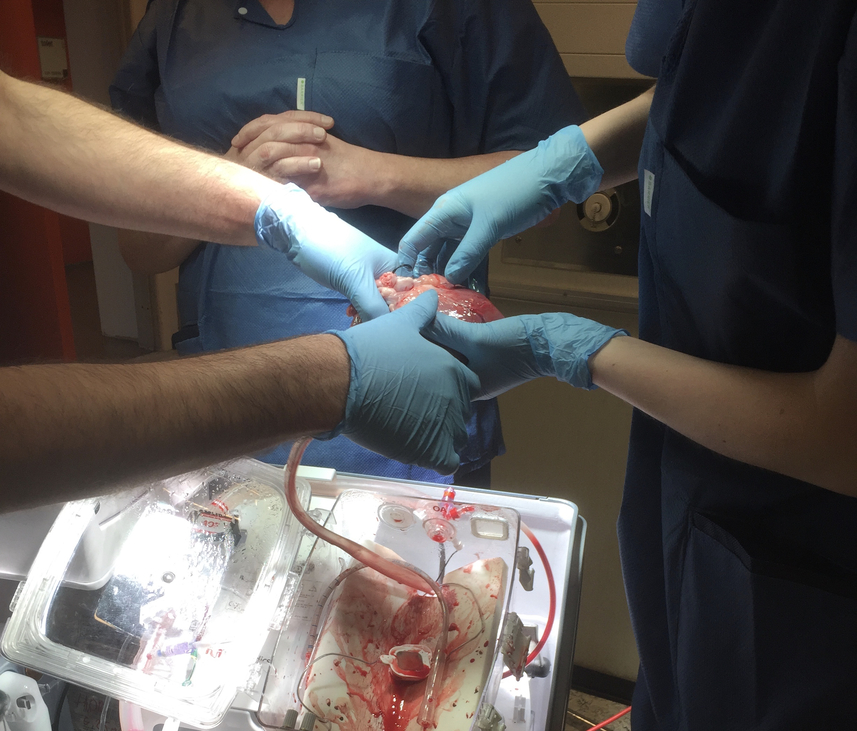
In the experiments we followed, the surgeons hooked the hearts and lungs of dead pigs up to so-called ex vivo perfusion machines that keep the organs ‘alive’ outside a body. Literally, this means that a heart can beat and a lung can absorb oxygen and remove carbon dioxide from within a machine. Ex vivo machines offer a technological solution to the problem of establishing and maintaining blood circulation in organs and monitoring and testing organ function. In layman’s terms, they are ‘lung-washing’ or ‘heart-washing’ machines.
In another experiment we followed, there were no ex vivo machines. Instead, surgeons deliberately damaged a pig kidney by storing it outside the living pig’s body for 26 hours, after which they injected it with stem cells (or no stem cells) and transplanted it back into the pig. Various capabilities and functions were subsequently monitored—for instance, the pig’s ability to urinate following the kidney transplant—in order to ascertain the effects of the stem cells.
The ambition of all above-mentioned experiments was not only to ensure readiness for DCD organs but also to ensure that ‘damaged’ organs from so-called ‘marginal’ donors otherwise unsuitable for transplant due to donor age or medical history could be ‘improved’ and safely considered. In these experiments, pigs modelled both human donors and recipients and were crucial for improving the pre-existing practices, realising current potentials, and paving the way to a future where all donated organs can be technologically or genetically improved.
Research animals have the potential to become epistemic things (Rheinberger 1997) holding collaborative value (Davies 2012) for the involved researchers. Previous studies on human-animal interactions in experimental science have shown that research animals are able not only to translate knowledge from laboratory to clinic, but are instrumental in coordinating and transforming social relations across disciplines and institutions (Dam et al. 2018; Nelson 2018; Friese and Clark 2012; Sharp 2019 & 2019a). This collaborative potential necessitates anthropological attention in the field of interspecies transplant research. In this paper, we investigate how pig experiments facilitate ‘collaborative intimacies’, which we define as social relationships across species and medical disciplines emerging as a result of close collaboration in laboratory work. In particular, we will focus on how these close collaborations provide opportunities for medical training and facilitate moral reflection and social networking among transplant professionals participating in the experiments.
Theoretical framework: Collaborative intimacies
In medical science, animal research has been fundamental in developing knowledge about the human body and translating knowledge from a laboratory setting to a clinical one. A number of social science studies have dealt with the infrastructures of such translational science by investigating the development of animal models, the circulation of technologies and knowledge between laboratory and clinic, and the relational aspects in human-animal encounters (Friese 2013; Davies 2012; Dam et al. 2018; Nelson 2018; Kirk 2016; Svendsen 2020). While rodent models are well established in biomedicine, pigs have become increasingly popular due to their similarities to humans in terms of anatomy, physiology, size, and genetics. Recent ethnographic studies of Danish farming practices and of research piglets in Danish neonatology have labelled the pig a ‘hybrid’ due to its position as simultaneously close to and far from the sphere of the human (Anneberg et al. 2013; Dam et al. 2018). In the laboratory, the transplant research pig is not fully ‘animal’, but can certainly never be fully ‘human’; it is appreciated because it spans the border zones of species and exceeds categories. Because of this status, the transplant pig, dead or alive, becomes a moral and medical educator and enables an extraordinary medical space which transforms the relationships between the people interacting with it.
Recent studies have documented the ways in which research animals and human researchers are constituted by their mutual interactions, which in turn shapes the knowledge that can be built from such experiments (Haraway 2008; Dam et al. 2018; Davies 2012; Friese 2013). Drawing on these ideas, our work focuses on how various medical professionals interact with pigs and with each other during transplant pig experiments. Interaction in such contexts is unquestionably directed towards generating knowledge within the framework of the research protocols, and yet such work also involves (whether deliberately or not) developing surgical skills, sharing moral reflections, and expanding social networks. When transplant pigs model as human patients, the social relations among transplant professionals are reconfigured.
Taking inspiration from studies of translational infrastructures (Friese and Clarke 2012) and animal intimacies and interspecies relatedness (Govindrajan 2018; Sharp 2019), we propose that the transplant pig obtains different kinds of status in the laboratory which enable a space where extraordinary social relations and collaborations among transplant professionals are able to unfold across disciplines and hierarchies. In recent work, the concept of ‘intimacy’ has been suggested as a way to rethink collaboration, interdisciplinary work, and moral and sentimental structures when scientists engage with animals (Friese 2019; Latimer 2013; Latimer and Gomez 2019; Sharp 2019 & 2019a). Our term ‘collaborative intimacies’ conceptually embraces how collaboration is practised in an experiment where the handling of the pig allows for particular forms of training, reflection, and networking among professionals. The social relationships that consequently arise among transplant professionals have the potential to reach far beyond the activities and parameters of the actual research experiment. We argue that these collaborative intimacies affect not only future knowledge production, but actually transform the career potential and ethics of the involved transplant professionals.
Methods
Ethnographic fieldwork was conducted between February and May 2018. Researchers observed and participated in experimental animal research in kidney, lung, and heart transplantation in two Danish university hospitals.
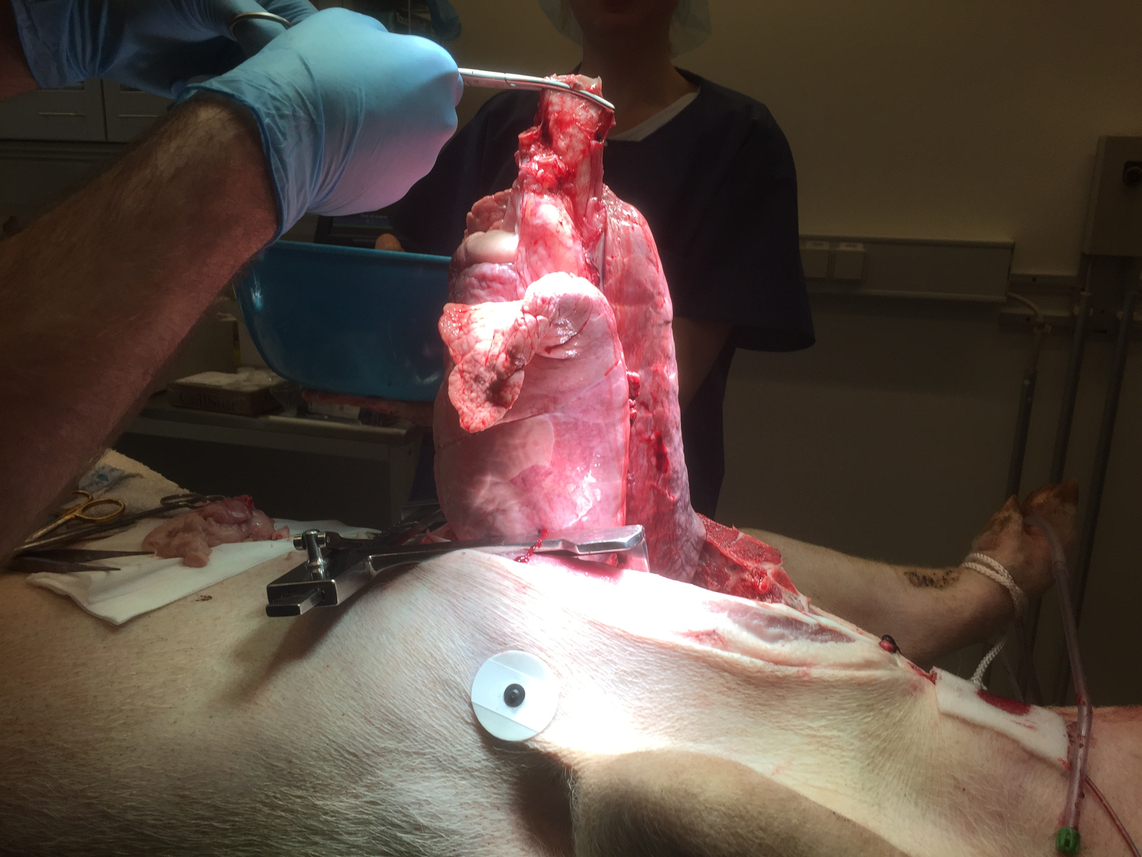
During our research into heart and lung transplants, we observed the killing of the pig and the ex-plantation (removal) of the heart and lung as well as the organs’ transfer to the ex vivo machines. We observed the ex vivo perfusion and the meticulous testing of organ functionality. In these experiments, the pigs are euthanised on the surgical table; scientific interest concerns the procurement of organs, the function of the pig organs while hooked up to the machines, and how the machines and perfusion solutions can be best adjusted to optimise organ function.
To observe the kidney research, Jensen went to the animal research farm and followed researchers’ and caretakers’ practices in the pig quarters and on the operating table. Unlike the observed heart and lung research, in this study the pig was not killed immediately. Instead, it had one kidney removed, which was stored and intentionally ‘damaged’ for 26 hours. The next day, the pig received its own kidney back after the organ was perfused with pig stem cells. This was in order to investigate whether and how the stem cells were able to repair organ damage. The newly transplanted pig was then monitored for a survival period of 14 days before it was euthanised, during which time the ability to produce urine and the overall wellbeing of the pig were systematically observed.
We interviewed 10 physicians and surgeons and one research coordinator who’d been overseeing the animal experiments. In addition, we had many informal conversations with transplant nurses, laboratory scientists, veterinarians, and animal keepers across the facilities. On top of this fieldwork, our study draws on insights from Jensen’s engagement with Danish organ donation and transplantation practices since 2008 (Hoeyer & Jensen 2011, 2013; Jensen 2009, 2011, 2011a, 2016, 2017; Jensen and Larsen 2020) and Svendsen’s work on human-pig relationships in biomedicine since 2010 (Dam et al. 2018; Dam and Svendsen 2018; Svendsen 2015, 2017, 2020; Svendsen and Koch 2013). This current project is part of Svendsen’s research project ‘A Life Worth Living’ (LifeWorth), which focuses on the value of precarious human and animal lives in the Danish welfare state.
The managing doctors responsible for research protocols at the Danish university hospitals in question approved our study, and all data was handled and kept according to the rules of the Danish Data Protection Agency. Interviews were transcribed and, together with fieldnotes from participant observation, were coded, and the interviews then underwent thematic analysis (Attride-Stirling 2001). The dominant themes were discussed among the authors and their colleagues and presented at various international conferences. Before publication, the article was shared with our informants so as to obtain their responses and comments.
The Danish transplant pigs and the pig laboratories
The pigs used in our research’s focal transplant protocols are ordinary pigs from the Danish farming industry, born and bred on Danish farms. In Danish medicine, there is a long tradition of using pigs in research; pigs are seen as promising subjects for generating future knowledge, health, and wealth (Svendsen 2017). The fact that Denmark’s meat industry processes roughly 13 million pigs at any one time leads the surgeons in our study to conclude that the Danish public does not object to the use of pigs in medical research; the pig is already publicly accepted as a resource for the human. In the research laboratories where our fieldwork took place, lunch boxes with traditional Danish pork dishes such as meatballs [ frikadeller ] and liver pâté [ leverpostej ] were routinely kept in the refrigerators and eaten by surgeons and nurses. No matter how many pig organs replaced human organs on the operating tables, pigs remained trapped in the category of ‘meat’.
This ‘animal use-position’ (Thompson 2013) turns the pig into an ‘ethical (non)issue’ (Wolf 2009; Svendsen and Koch 2013) for the medical staff in the experiments. Anatomically and ethically, the pig is well suited to use in research. While other countries such as the United Kingdom and the United States have multiple animal rights organisations fighting against the use of animals in both medical research and the meat industry (Sharp 2019), we have encountered no protests in Denmark regarding the use of pigs in transplantation research. Some of our informants associated this lack of public criticism with the pig’s ingrained status as food in Danish culture, explaining that, in contrast, some of their international colleagues who are also involved in pig experiments had been attacked in their homes by animal rights activists. Despite this comparatively safe climate, the address of the animal research farm was kept deliberately obscure on associated university websites and even on Google Maps.
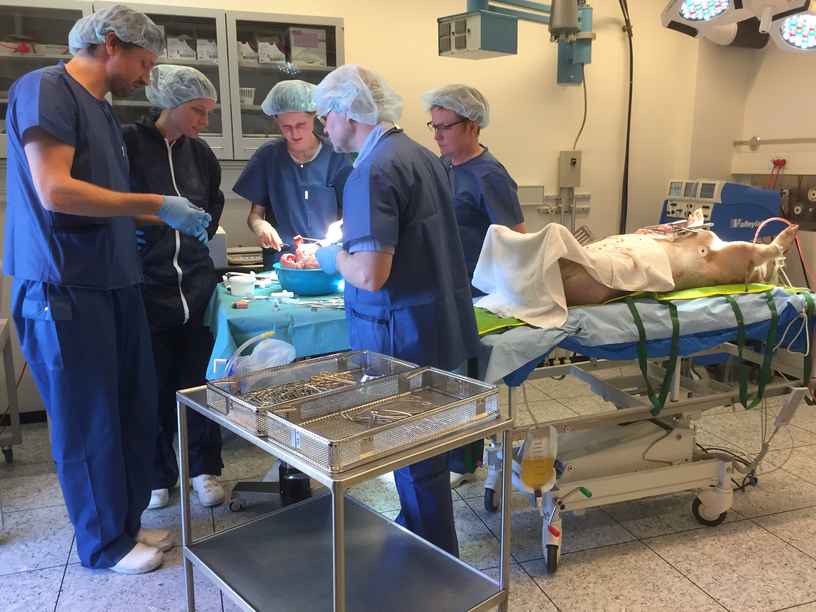
The experiments we witnessed took place in clinical laboratories at a university research farm and at a research unit at a university hospital set up to resemble as closely as possible a regular operating theatre for human patients. The kidney transplant environment was sterilised because the pig, like a transplant patient, should have optimal conditions for survival. Conversely, research laboratories for heart and lung experiments are non-sterile; doctors wear surgical clothes and sometimes gloves, but they do not wear masks or scrub their hands as they would for human surgery.
When the pig is placed on the table, it is with the sole purpose of delivering either heart or lungs. The lower part of the pig’s body acts as a table for the surgical instruments, and tubes and cords are held by the pig’s own cloven hooves. Using a patient as a table would never happen in the clinic. Moreover, in the pig laboratory it was not unusual to see a coffee cup next to the research protocol documents. The breaks between drawing and measuring blood from the organ currently on the machine were often spent chatting and—at a table near the theatre—eating pastries and discussing best practices and future hopes regarding the experiments. During our participant observations, surgeons and research coordinators frequently explained that the experiment was staged exactly ‘like real surgery’. Yet on other occasions they claimed the procedure was ‘far from a real surgical operation’. As such, we became curious about when and how the pig can be so fluidly moved in and out of frameworks denoted as ‘real surgery’ and ‘far from real surgery’. ‘This is so nice and relaxed compared to real surgery,’ Benjamin, the research coordinator in charge, often said, adapting his cosy and mellow attitude depending on the visitors and veterinarians walking in and out and commenting ironically on the presence of iPhones and coffee cups. When we asked Benjamin about this ‘loose’ attitude in the surgical laboratory, he replied:
Well, the pig doesn’t have to survive. So if there’s a little infection or bacteria in the surgical field, it’s no problem. We only need the hearts and lungs for some hours. So we stand there with our coffee cups and are not sterile. The clothes, the attitudes are more relaxed. We have more fun, I think . . . But still, it’s really serious and we do our best. But there are no human lives at stake. And you can feel that. It’s not a human lying there; it’s a pig.
How can the pig substitute for the patient while being, in form and practice, completely different from one? On the one hand, the pig mirrors the human patient because the organs, the vessels, and the procedures are alike. On the other, this surgical procedure is as far from human surgery as possible: the life on the table has no intrinsic value as an individual. This assessment spells out the identity of the pig as a substitutive research subject performing as something biologically similar to the human, but morally different (Thompson 2013). In the words of Lesley Sharp, ‘Animals are simultaneously expendable and extraordinarily valuable’ (Sharp 2013: 46). This duality means that the transplant pig possesses extraordinary power to facilitate extraordinary relations between the humans surrounding it and, as we shall see now, an optimal space for medical training in surgery and care.
Surgery and care: Medical training in the pig laboratory
In Danish experiments with pigs, transplant surgeons bring research forward, creating usable results by measuring organ function after reperfusion on ex vivo machines (for heart and lung transplants) or injection of stem cell solution (for kidney transplants). While the results indicate the potential to decrease organ damage and improve transplant efficacy, the pig experiments also allow for surgical training without risk to human life. Junior doctors practice their surgical skills during animal research experiments and are often made responsible for portions of the surgery. Opportunities like these are rare during ordinary surgical training, so the experimental space is, for younger or early-career professionals, important beyond the confines of the research protocol. The research space also offers other opportunities for knowledge that have the potential to improve future patient treatment. We interviewed Eva, an experienced transplant surgeon (in charge of the kidney protocols), who articulated the multifaceted purpose of the pig experiments:
The pig has a double purpose: you can make something that hopefully will bring us forward [help us progress] and you can investigate something which means patient treatment will improve, or we figure out some basic mechanisms that we can explore along the way. We make sure to include the younger surgeons so they get surgical training—meaning, they do not take their surgical baby steps with your neighbour’s girl. It’s a pig. Which after all, if it fails, is not so disastrous as if it were human surgery.
These ‘surgical baby steps’ are crucial for young doctors and medical students, and participation in pig experiments is in high demand among students. Malene, a young medical student (and lung surgeon) chose intentionally not to brag in public about her participation, fearing her peers’ jealousy. Malene was in her mid-twenties, in the later years of her medical studies. During participant observation, we noticed not only her surgical skills, but also her authority at the operating table and ability to educate visitors (and anthropologists) about anatomical details, the specificities of the research protocol, and the technicalities of the ex vivo machines. In interviews, Malene talked about an incident in the laboratory when they had to discard research findings due to a mistake in the mixing of the perfusion fluids that go through the organs hooked up to the ex vivo machines:
It’s a waste of resources. It’s not good. But, then again, it’s a risk-free environment. You haven’t caused any other harm than money, right? It’s too bad . . . it’s our time and our money, but we’re also wiser now. And it didn’t cost any lives. The thing about pigs . . . I’d never be allowed to jump into some research project, when I don’t have any skills, if it hadn’t been performed on pigs. You really learn so much from it over there [in the laboratory].
The experimental space of the pig experiment constitutes what experienced professionals frame as a surgical ‘playground’ where Malene and other future surgeons can prepare for the day they will be faced with a human patient on the operating table. Here, they can take their ‘surgical baby-steps’. In Malene’s account, the research laboratory is a ‘risk-free environment’, offering plenty of learning opportunities in which the pig resembles a patient, yet does not carry the value of a human life.
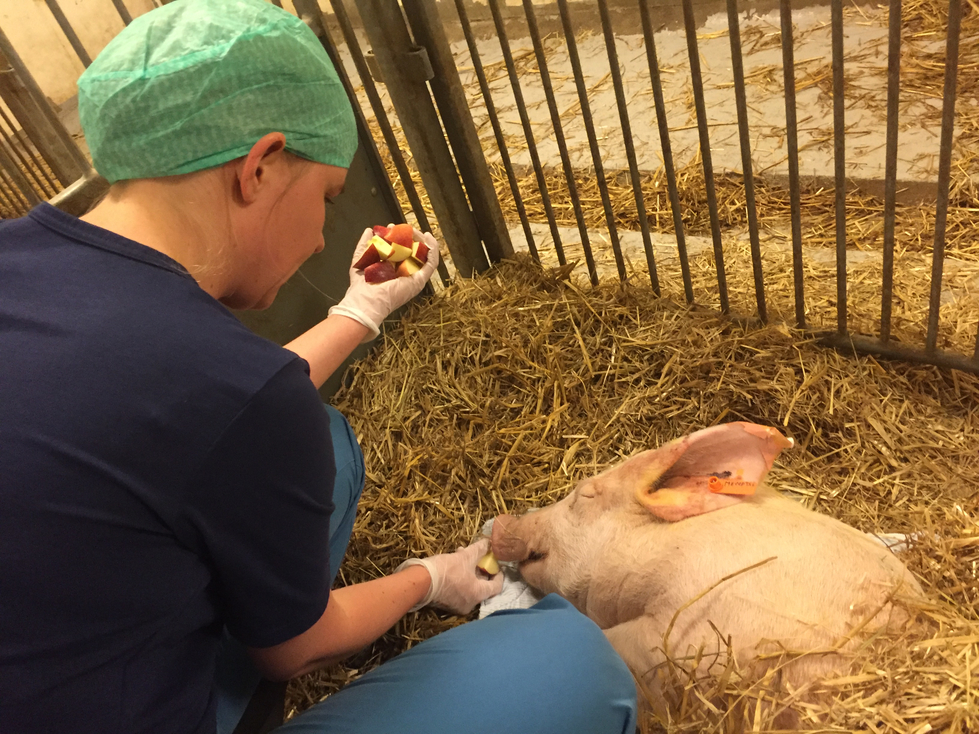
Surgical skills are not the only thing honed by the pig experiments; junior doctors also become familiar with the experience of caring for a fragile being through their interactions with the pig. During fieldwork, we noticed that timeframes played a significant role in the human-animal relationships that developed. Pigs that were euthanised right away (in heart and lung experiments) were not subject to the same attention from surgeons as the pigs that underwent kidney transplants, which were monitored for a period of 14 days after they received the kidney. On the small farm, 30 minutes by car from the university hospital, animal keepers and junior doctors took care of the pigs. They trained them to lift their legs so blood and urine could be drawn, and fed them apples to see whether they were able and/or eager to eat. These interactions had the primary purpose of turning the pigs into good research subjects—pigs treated in this way became calmer and more comfortable, and thus more willing to co-operate. However, as numerous studies of care in animal laboratories document (Friese 2013; Druglitrø 2016; Svendsen and Koch 2013), such contact also affected how the surgeons cared for the pigs. When operating on a cared-for pig and returning it to the stable, surgeons often took the time to chat to the other pigs, scratch them behind the ears, and feed them apples while chatting with the animal keepers about their general condition.
During the experiments at the university hospital, the kidney pigs wore socks to stay warm, had lotions applied around their eyes to treat redness, and their blood pressure and pulse were constantly monitored. The care practices constituted collaborative intimacies with animals (and also with veterinarians and animal keepers), which simultaneously improved the outcomes of the protocol and taught medical students about care and cross-disciplinary relations more generally. Eva, the kidney transplant surgeon, said:
It’s a funny thing: When you’re responsible for another living being, be it a child, be it a pig, you start developing emotions for it. You automatically start looking after it. Also, the pigs need a certain body temperature. They mustn’t be cold. Actually, there’s this unconscious ‘care-gene’ being activated when you look after somebody. It’s a basic reflex to take good care of them.
Even though pigs in transplant experiments are used as research animals with the sole purpose of providing organs and bodies to contribute to the development of medical science, surgeons like Eva, along with her medical students, worked together to provide care for the pigs and, as Eva explains, they felt some affection for them. This relates to larger anthropological debates on the practices of care, the collaborative dimensions of care, and caregiving as a defining moral practice making caregivers and receivers more human (Kleinman 2009; Mol 2008; Mol et al. 2010). The interspecies relationships that develop between researchers and their animal subjects have been thoroughly discussed by Lesley Sharp in her studies of American animal research, where lab animals were often given nicknames and were remembered and even mourned after their deaths (Sharp 2013 & 2019). In another Danish context (Dam et al. 2017) where research piglets were used as a gateway to generate knowledge on neonatal feeding practices, piglets were named, which, while individualising the animals, momentarily turned them into subjects with sentience. Lena, a medical student in the kidney experiments, said:
Well, I find all pigs adorable, and I always try to give them as much love as possible, like scratching their ear. Well, you really care about the pig. It’s a life— until we put it down of course. And I really like animals. I cannot handle it as if it were a steak or something. We’re very gentle, nice and easy. We don’t talk too loud when the pig is in the ward, because it shouldn’t feel stressed. We want the pig to feel good and we’re very aware [sic] that it’s properly sedated and it won’t experience any pain. You constantly check pulse and blood pressure to observe how the pig is doing.
In the research setting, caring for the pigs is something scientific (the well-being of the pigs is closely connected to scientific outcomes) and something relational (surgeons like the pigs). If a pig is not calm or is distressed, increased stress levels might affect its ability to endure surgery or prevent its post-surgical healing, thereby negatively influencing the outcome of the research protocol. Paraphrasing Carrie Friese and Adele Clark (2012) (more specifically, their ethnography of animal laboratories in reproductive science), care is performed primarily in order to optimise research and generate skills for future transplantations—however, it also teaches medical students about patient care and how to handle emotions and relations in clinical practice, something that also dominates the everyday working conditions of health professionals in the transplant clinic (Jensen 2017). In the intimate care space in which the transplant pig moves between animal and human classification and thus the categories of expendable and valuable life, it becomes a perfect educator.
Death and donation: Moral reflection in the pig laboratory
In post-mortem organ donation, the death of the donor is the starting point for saving or improving the life of an organ recipient, thereby exposing a delicate dichotomy between the utility and dignity of human bodies (Lock 2002; Sharp 2006; Jensen 2016). This has sparked many ethical discussions regarding the clinical practices of donor detection and the art of balancing end-of-life care, donor management, and organ optimisation (Forsberg et al. 2014; Hoeyer and Jensen 2011; Jensen 2011). In transplant experiments, the pig does not die like a human donor. It is killed in the name of science in order to provide knowledge. Michael Lynch’s (1988) study of a neuroscience laboratory uncovered the transformation of the ‘naturalistic animal’ into an ‘analytic animal’ from which scientific facts can be generated and transferred into the clinic. Thirty years later, Lesley Sharp (2019) illuminated the moral reflections and responses of the lab researchers and animal technicians facilitating this transformation: they care for animals, work with them, and in the end kill them. During our field studies, we experienced how the pig experiments and the necessary deaths of transplant pigs resulted in moral reflection and transformed the laboratory into a moral space (Mattingly 2014) for handling and reflecting on death. We therefore argue that laboratory pigs bear pedagogical resemblance to human donors in anatomical dissection, who have been described as mentors or teachers for medical students (Douglas-Jones 2017; Olejaz 2017).
Unlike medical training and surgical procedures involving human subjects, it is a basic premise of the pig experiments we followed that the pig is killed immediately before surgery (for heart and lung protocols) or after being monitored for a period of 14 days (for the kidney protocol). According to Michael Lynch (1988), the killing is what transforms the laboratory animal from naturalistic animal to analytical entity and bearer of generalised knowledge. Unlike death in a human clinic, the death of the pig in the laboratory is inevitable, desired, and carefully planned. When asked about their feelings regarding the termination of the pig after the recovery period, in which blood and urine samples are meticulously collected, kidney surgeon Eva explained:
It’s an important day of celebration: you’re able to collect all your data based on huge efforts. At the same time, it’s also sad. You develop a personal relationship with those pigs. You’ve been taking care of them, so there’s the dilemma of killing them. But that's how it is. I think it’s a mix. You feel empty. It’s perhaps not the best example, but somehow like the way you feel after an exam. You’ve focused a lot on something, and then it’s over. It’s not sad because although you feel bad for the pig, it dies in a good way. But you have some kind of connection. You have a kind of emotional relationship with that pig, even if it sounds silly. It has its charm.
In short, killing marks the celebratory end of the experiment but also represents a certain emptiness related to the care practices and emotional challenges that constitute a fundamental part of translational research and human-animal encounters (Dam et al. 2018; Sharp 2019; Svendsen 2017).
When following the kidney studies, we met Sally, a PhD student and junior doctor wishing to become a kidney surgeon. She commuted happily between the university hospital and research farm, bringing medical students who assisted in monitoring and caring for the pigs and keeping track of the data produced in the studies. Visiting the farm, we noticed the large green trash can for the disposal of the pig body after the end of the surgical protocol.
When talking to Sally about her experience of terminating pigs’ lives and the broader matter of killing, she explained:
It’s weird. Because you’re killing the patient. That is different. That’s really an aspect that is different from the [human] clinic. Ending the experiment with killing the patient. That’s not something we train for. It’s okay to give the injection so it dies. But it’s really weird throwing it in the green trash can, actually. Because that’s when you hear a big ‘bump’—and it lies, rather unsightly, in the trash. Then it has become real garbage. . . . I make sure to throw it in headfirst so I cannot see the head. Because if it’s lying there in the garbage, totally dead, with eyes open, looking back at me, that is very weird.
Sally does everything according to the research protocol. The life of the pig has to be terminated and its body put into the trash can. But by ‘looking back’ at the surgeon from the trash can, the pig is no longer a substitute for the patient, nor a farm animal providing meat, but somehow inhabits the moral space of the human patient. The pig’s final destination, the trash can, is not comfortable to Sally; and the pig she has been closely monitoring for two weeks before and after surgical interventions, should not, when dead, look back at her.
Sally’s contemplations bring into focus the morality of the termination of the pig and the disposal of the pig’s body. Why is it ‘okay’ for Sally to give the injection, but strange that the pig ‘looks back’ at her from the trash can? Moving across and between the categories of patient and animal, the pig acquires the potential to leave a significant trace on the education of doctors with regard not only to stem cell solutions for organs, but also to patient care and moral behaviour in end-of-life matters. To quote John Berger (1990; 14, 25 in Sharp 2019, 1), ‘the animal does not reserve a special look for man . . . man becomes aware of himself returning the look.’
Even in death, Sally’s intimate relationship with the pig (whether the body went into the bin headfirst or not) turns the animal into an educator regarding death. When facing the pig, or when choosing not to face the pig in the trash because she finds it ‘weird’, Sally, we believe, contemplates her dual role: at once she is a carer and death-bringer. She is simultaneously educated about her future role in life-and-death decisions; the associated need to act correctly; the moral aspects of medical experiments; and how to handle the emotional aspects of medical practice. When the pig is killed and its body is disposed of, the laboratory likewise becomes a moral observatory for researchers: death leads researchers to start reflecting on the morality of their actions (Sharp 2019). Reflecting upon death is an important skill for a doctor, especially within organ transplantation, where the death of the donor is a prerequisite for transplantation, to say nothing of the fact that transplant surgery is a high-risk procedure for recipients and that post-transplant recovery is not always successful. Transplant professionals inevitably work in the presence of death (Jensen 2017).
The transplant professionals we interviewed admitted to often contemplating the death and suffering of the pigs in relation to both animal welfare and to donor surgery. Eva, the kidney surgeon, remembered as a young doctor going to a slaughterhouse for some blood for a research project and being terrified to see pigs screaming in fear as they entered the passage where they would be killed by carbon dioxide. Knowing that her own experimental surgical practices also involved killing pigs, she explained:
I know the pig has to be killed. But if we didn’t bring it here, it would also die and end as pork roast [. . .] I’m so happy it’s received pain relief or been anesthetised when I was doing something that would have otherwise hurt it. I know it gets an overdose and leaves this world on a pink cloud instead of screaming from fear in the carbon dioxide grave. In the way it ends its life, it’s done good, it hasn’t suffered, and, regarding the very last minutes, this is a better alternative.
Eva clearly thinks it is better for the pig to die on the surgical table than in the slaughterhouse. She even considers the pig as having ‘done good’, thereby implicitly tying the pig into narratives of human organ donation (i.e., the common association of human organ donation with heroic sacrifice).
In the heart and lung experiments, as soon as the heart and lungs were removed, Jensen noticed how the dead pig was ‘left alone’ to wait for the veterinarian to arrive and discretely throw the body into the trash can. The researchers’ attention was continuously on the organs, and their surgical expertise was centred on maintaining organ function and perfusion on the ex vivo lung or heart machine. Jensen could not help noticing the sudden shift of attention away from the pig, as everyone’s eyes followed the organ to the table where it was prepared for the machine. The body parts were the new centres of attention, replacing the body from which these parts were taken. After witnessing this shift in attention on the first day of fieldwork, Jensen, heavily influenced by years of studying human organ donation and the perspectives of donor families, spontaneously asked if the pig should not be stitched together again. The research coordinator Benjamin smiled and said, ‘You’re so sweet—why would we do that? For the sake of the pig? Or so its family can come and say goodbye?’ Realising the naivety of the question, Jensen asked if they ‘would use the pig for anything else’. Benjamin laughed and said, ‘Yes, we’re considering having a pork feast [ ‘grisefest’ in Danish], but Samir here is not so much into the idea.’ Here, he pointed to the junior cardiac surgeon, who was a Muslim. Smiling back, Jensen realised that the body of the pig was no longer of any value, nor was it any longer an object of surgical interest. It was simply trash, unworthy of attention or, as Benjamin’s joke indicated, food. A month later, we mentioned this episode to Carl, the head cardiac surgeon we were interviewing. He smiled at us and said:
That’s actually not too crazy. I remember at first [doing this] many times; I also stitched the pigs together before they were thrown into the trash. Also [this was] in order to keep it all together in the body. But I realised it was a waste of energy, because it was just wrapped in that bag and thrown in the trash can.
The pig becomes garbage after it has done its duty. But, somehow, this had felt wrong to Carl—he didn’t like the idea that the body on the table was left and discarded in a way that would never happen in a clinic.
However, while the differences are stark, the bodies of human and pig donors are not unconnected; critical social science studies of human transplants have pointed to the instrumental use of the bodies of human organ donors (Fox and Swazey 2013; Lock 2002; Sharp 2006). Kidney surgeon Eva, in conversation with Ellen, a professor in nephrology, said:
The thing where the heart is removed from the pig. Looking down at it, it’s like a house where a bomb has fallen. The walls are still there, but everything is ruined inside. The feeling of catastrophe. It’s different [from our practice of kidney surgery].
To which Ellen responded:
Somehow it reminds me of watching a deceased human organ donor having organs removed. That’s also disturbing somehow. I have participated a few times [in human organ procurement]. You get in a really sad mood. Because you can still see that this used to be a human being until very recently. And now it’s lying there, completely empty. You can see that it makes sense, but you have to handle it with grace and sensitivity.
The pig’s body reminds Ellen of a human donor’s body. Empty. Disturbing. Yet, in the clinic, the emptiness makes sense because the organs will help others by being placed immediately into the patients awaiting transplants. The pig organs operate in a different temporality regarding patient outcome: they contribute to research that might, in the future, improve organ function and widen the pool of human donors. Jensen asked if the shift in attention from the pig to the organs also occurred during a human organ removal. She replied:
No. It’s completely different. [In human organ removal], you’re very respectful. There are always two surgeons at an organ removal. One of us focuses on the kidneys and takes care of perfusion. The other one helps with the surgery and makes sure to close [i.e., stitch up] the donor patient. Once, it was a junior doctor and the stitches were a bit clumsy and sausage-like. And I didn’t think that was good enough. So I have now made sure that the same stiches are used on donors as if it was real surgery. So the donor looks as nice as if it was a real surgical operation where the patient [later] woke up. Otherwise, I would find it unethical. It has to be done nicely. I don’t think anybody will rip off the patches, but if they do, it has to look like an ordinary surgical procedure.
Eva’s reply underlines the intersection between care, dignity, and aesthetic practices in donor surgery (Jensen 2011), marking the ethical boundary between the two species. The pig is left to itself after organ removal; it is disposable and nobody will notice how it looks after surgery. The ‘empty’ human donor’s body, however, carries different meanings. It is not ‘left alone’ and has to look nice, else the kidney surgeon finds it ethically improper.
Similarly, Sonja, another kidney surgeon, while discussing the practices of sterile surgery, explained:
After the organs are gone and the patient is being closed [stitched] up, you still work in a sterile environment. Unlike the pig experiments where, if you drop some instrument on the floor, you pick it up, [with human donors] you would never reuse it. Even if the donor is dead. It has something to do with the respect for the task you have.
The rather ugly scene of the open pig body—dirty instruments and leftover tissue placed on the body, instruments picked up from the floor—constitutes both a contrast and a similarity to the process of human organ procurement. Seen from a utilitarian perspective, the human body, like the pig body, has no worth once it is emptied of organs. Nevertheless, for families and staff, the empty human body holds value and needs to be respected as if it was a living person (Jensen 2011). To maintain the ethical standards of organ procurement, Eva makes efforts to handle donor bodies like other surgical patients. What separates pig experiments from donor surgery is the caring of others connected to the deceased human body: the grieving family members and the dedicated transplant professionals well aware of the donor’s social relations. The human donor’s body has an audience, a body of people who care about how it looks—who want to say goodbye. The pig’s corpse, on the other hand, is left on the table for the veterinarian to place into the big green trash can for disposal. Nobody cares about the surgical field being left open; nobody will see this body again. Nobody is grieving. The Danish transplant pig does not gain the status of some research animals in American laboratories, which leave laboratory personnel grieving and even inspire memorials (Sharp 2019). To the clinicians involved in the transplant experiments, it is not the empty pig in itself that matters; the emptiness comes to matter because it challenges clinicians’ perceptions of what constitutes good and respectful conduct around donor patients. The pig experiments, both in contrast to and similarly to human organ donor surgery, make the professionals involved reflect on life and death and articulate and define the border zones between caring and using, respecting and ignoring, which lie at the centre of human organ transplantation. Pig experiments initiate moral reflection and in so doing ensure that clinicians treat human organ donors respectfully, even after death; as such, the experiments become a moral ‘testing ground’ or laboratory (Dam & Svendsen 2018; Mattingly 2014; Sharp 2017). The pig experiments thereby reconfirm the structure of the clinic, where organ donor patients are treated with respect, sutured nicely, and touched only with sterile instruments, even if the morgue is the next stop. To clinicians and researchers involved in pig experiments, the sometimes intimate and collaborative relationships formed with the doomed animals suggest pig research studies act as moral observatories for looking at and reflecting upon how organ procurement and donor bodies are handled in the clinic. The collaborative intimacies bring about moral reflections.
Advancement and hospitality: Social networking in the pig laboratory
Pig experiments provide a platform for many kinds of cross-disciplinary collaboration, welcoming researchers from both medicine and social science with well-defined research agendas (Dam et al. 2018). We now show how the transplant pig laboratory offers social networking opportunities for medical professionals in distinct and unique ways.
In pig experiments, doctors, nurses, and students participate actively in various research protocols, performing tasks outside their usual professional remit: doctors clean surgical equipment (normally a task for orderlies and nurses) and medical students perform surgery. The experimental setting, where the pig ranges from patient to waste, enables a transformation of the traditional hierarchies of medical education—an important aspect of what we frame as collaborative intimacy. The pig research coordinator, Benjamin, explained:
The professional boundaries are erased once in a while. As a nurse, you perform some surgical tasks. As a doctor, you perform some nurse tasks. Or lab tasks. We don't have so many resources. We have to do it all. Also, the cleaning of equipment.
Junior surgeons become acquainted with the work, routines, and responsibilities of their colleagues when performing tasks not usually assigned to medical doctors. Removing hierarchies and professional roles is a way for them to be introduced to the different tasks involved in surgery. The medical PhD student, Sally, said:
For this kind of research, you have to think it all through yourself. What instruments to use, the size of the intubation tube—you have to make many decisions. In a way, that’s really good, because if you’re going to be a surgeon someday, which I might be, then you get a better understanding about the other professions—what it means to intubate, what it means to be sterile and prepare an operating room before the patient arrives.
In the pig laboratory, it becomes acceptable to rehearse and negotiate the different roles and functions of the medical hierarchy. As such, the laboratory becomes a space where medical professionals create and maintain social networks that might provide, in addition to medical training and experience, future access to and advancement in the clinic. Benjamin explained:
You make a deal with medical students. You get their help and they get a small salary, but in return they get surgical experience. And they’re very eager to join—we almost cannot keep them away because they really want to. And that’s the way they get both surgical experience and contribute to scientific journal articles. This is as good as it gets for someone like Malene, who wants to become a surgeon.
In this way, the material relations of handling instruments and operating on pigs engender a connectedness between the human actors collaborating in the experiment while at the same time preserving differences between senior and junior colleagues and between species (Latimer 2013). Through these collaborative intimacies, access to surgical training is offered up in exchange for working hours. This is not new in the history of medical education, but we argue that the level of involvement and responsibility is possible in the laboratory only because the ‘patient’ is a pig; therefore, making a mistake does not harm human lives.
Shortly after our fieldwork was completed, ex vivo technology was used for the first time to transport a human heart from a donor in one part of Denmark to a recipient in another, the ex vivo machine being similar to, albeit newer than, the one used in the pig experiments observed at the university hospital. This was relatively innovative: the ex vivo machine enabled longer ischemia time (the period that the organ can ‘survive’ without oxygen), meaning organ perfusion could be preserved during transportation without the need to keep the heart on ice. Taking care of the human heart in the ambulance was Samir, the young doctor who conducted the pig experiments where pig hearts were put on ex vivo machines. Given his experience of the pig laboratory, he was familiar with the equipment, the solutions, and the other technicalities of machine-enabled organ perfusion and, as a result, was trusted by leading heart surgeons to play this vital part. While details regarding the transplantation were withheld, we were told that the recipient of that particular heart recovered extraordinarily well soon after the transplant. Stories like this give us reason to predict that, when Denmark starts using organs from DCD donors and reaches the point where machine perfusion of human organs becomes common, researchers and surgeons with experience of pig experiments are likely to be seen as key candidates due to their fundamental knowledge and experience of the technology. As such, pig experiments offer tremendous potential for career advancement in the field of human transplantation.
After our fieldwork, Jensen attended a European transplant conference where she met Ellen, a professor in nephrology. Ellen explained that she was frequently rushing back and forth to meet with future collaborators regarding new projects. ‘I just met with somebody from Holland,’ she said. ‘It’s actually because of our collaboration in the pig experiments that we’ve built this new network.’ As this makes clear, we need to consider the potentials for networking when speaking about collaborative intimacies, which are themselves enabled by the attributed status of the transplant pig. These potentials are valuable even for very experienced and established researchers working, like Ellen, towards expanding existing international networks and funding opportunities.
In contrast to the secrecy and closeness surrounding human surgical practice, it was, we observed, a very prominent and highly valued feature of pig experiments that guests were welcome. Benjamin, the research coordinator in charge of the practical execution of the heart and lung experiments, was quite the narrator and educator when it came to showing and explaining the procedures and the contents and intentions of the research protocol. Often visitors joined the experiments, especially nurses from the hospital’s cardiac intensive care unit who, Benjamin said, wanted to ‘learn about the ongoing research’ and ‘get some anatomical teaching up close without having to stand quietly in the background’ (which they’d have to do in clinical surgery). Benjamin also invited guests to touch the heart and the lungs while they were attached to the ex vivo perfusion machine. He explained, ‘People who visit are humble and used to showing a certain respect and quietness [in the human clinic]. I try to loosen [things] up and say, “Come up close, let’s learn something, see something, and touch something.”’
Coordinator Benjamin’s descriptions of his interactions with guests illuminate how the pig effortlessly moves between categories: in this case, between the clinic and the laboratory. Its medical value thereby lies not only in enabling the success of research experiments, but also in enabling intensive care nurses to get close to the surgical field and for other ‘outsiders’ to visit, learn, and be inspired by the research experiments. Visitors, including Jensen, were sometimes invited to step into the surgical field to hold the suction pump and even cut some of the major vessels when removing organs from the dead pig. It was also completely acceptable that both surgeons and visitors were allowed to take photos and short film clips to ‘flash on social media’.
‘This is a real Instagram moment,’ Benjamin would say as Jensen admired and photographed a beam of sunlight from the window hitting the pig heart still beating on the green cloth of a surgical table after having done its research duty for four hours on the ex vivo heart perfusion machine. The pig as patient demands no privacy; it does not need to be protected from visitors.
During our fieldwork, we noticed how the experimental setting became an emotional refuge where the challenges and difficulties of the medical profession could be articulated across professional boundaries between both transplant professionals and visitors. One day, standing around the table where a pig heart was being prepared for the machine, the visiting intensive care nurses and doctors began discussing a night shift during which a two-year-old boy had been brought in, lifeless from having choked on some grapes while sitting in the rear car seat on a trip to the carwash with his grandfather. Due to the noise of the carwash, the grandfather had not heard the screaming and choking, and when the boy collapsed in his seat, the grandfather thought he was sleeping; in reality, his heart had stopped. ‘They discussed donation when my shift ended,’ the nurse said. There was silence. Then, Benjamin went back to talking about the pig experiment and the next step in the protocol, joking about this and that while the pig heart was placed on the ex vivo machine and started to beat nicely and regularly, all according to protocol. Along with the question of surgical sterility, the graveness of life and death and the destinies handled by health professionals constitute a major difference between the research setting of pig experiments and that of the clinic.
In the research setting, the pig comes to represent a donor free from the pain and tragedy that are inevitably associated with human donors and patients. In contrast to the emotional challenges surrounding professional life at transplant units and of caring for organ donors and families (Jensen 2011, 2016, 2017; Sharp 2006), working with pigs provides a much-needed emotional refuge. When there is no human life at stake, the sharing of emotions somehow becomes possible and acceptable, and stories of suffering from the clinic can be brought in and shared because there is at last the time and space to do so. The investigative space of animal experiments thus represents a way of navigating between life and death without pain, and offers new opportunities with regards to career progression, medical advancements, and the maintenance of good mental health. The pig cannot meaningfully model the loss of life usually associated with organ donation, and thus reconfigures existential loss into new practical options for surgical and technical improvements and for sharing emotional stories, thereby establishing collaborative intimacies.
To sum up, the pig experiments enable social networking on many levels. They provide a platform for career development for young professionals—a place to be, to be seen, and to be acknowledged—as well as a springboard for new international collaborations and funding opportunities for experienced professionals. Experience in the pig laboratory is, in itself, a potential ticket to career advancement in surgery. Equally, it is a welcoming, relaxed environment freed from the clinical hierarchies associated with human surgery. Interested visitors are encouraged, and are typically eager to make use of the rare opportunity to experience surgery up close. In addition, the pig provides a refuge in which there is time and opportunity to articulate the emotional difficulties of the human clinic.
Conclusion
We have shown how using a pig as a model for a patient establishes collaborative intimacies among transplant professionals and sometimes also among professionals and pigs. These collaborative intimacies are shaped through the socio-material relations of experimenting with new technologies, caring for the pigs involved, contemplating death and donation, and standing alongside other more senior or more junior professionals. As such, our ethnography supports the ambition to free intimacy from its association with the private and personal to instead uncover the significance of intimate work in more-than-human relations (Latimer and Gomez 2019). In particular, our ethnography demonstrates how the pig, and in particular the status attributed to the pig in these experimental settings, enables collaborations where knowledge is produced and the medical and social futures of transplant professionals are shaped.
Certainly, the ongoing need for human organs will allow for several future transplant imaginaries (Sharp 2013) where surgeons and researchers will seek to improve available human organs, design and grow new ones through stem cells, and include other species as potential donors. We suspect that future scenarios as to how animals become blended with humans in frontier medical science and clinical practice will continue to call for reflection and conceptualisation via social science (Rémy 2009; Sharp 2013). Our message is to pay attention not only to technological progress, but also to how the intimate relations, which are an inherent part of research into new technologies, matter for medical professionals in the future.
We have proposed collaborative intimacies as an empirical and theoretical framework for conceptualising and re-imagining the social implications of translational work. In transplant research and in other kinds of experimental medical science, we encourage scholars of social science to focus their attention on the collaborative intimacies—that is, the kinds of togetherness—that arise in experimental environments. Research experiments are not only about reaching new levels of knowledge; as we have shown, experimental settings establish among health professionals social relations that are essential for educating future generations of surgeons, for practising moral reflection, and for building and maintaining social networks that will shape medical futures. In these knowledge-making spaces and during the experiments they’re home to, we suggest attention is paid not only to the ethical dilemmas arising from technological progress; rather, the collaborative relations that form between the medical professionals participating in the pig laboratory represent a fundamental key to render visible the broader moral and technological horizons of current clinical practices and future medical fields.
Acknowledgements
We would like to thank all informants at the university hospitals for welcoming us into the pig laboratories and for participating in our research. When presenting our initial findings prior to this article, we benefitted from inspiring comments from the participants at the Valuing Health conference at the University of Edinburgh, the Laboratories of Health workshop at Kings College London, the CRESIDA seminar at the University of Roehampton, and the Practices of Substitution panel at the AAA in San Jose. Special thanks to Lesley Sharp for the continuous inspiration, dating from the beginning of this project, and to Mie Seest Dam, three anonymous reviewers, and the editors of MAT for their careful readings and valuable comments. The research was funded by Mette N. Svendsen’s Sapere Aude grant from the Danish Council of Independent Research (12‐133657). All photos in this article are by author Anja M.B. Jensen.
About the authors
Anja M. B. Jensen is associate professor of medical anthropology and co-director of the Centre for Medical Science and Technology Studies at the Department of Public Health, University of Copenhagen. Her research focuses on organ donation and transplantation in Denmark and the USA and the intersections of medical technology, public policy, and human emotion. She is particularly devoted to exploring the experiences of donor families, medical staff, and organ recipients, as well as the ethical issues of organ donation and, most recently, increased data sourcing in transplant medicine.
Mette N. Svendsen is professor of medical anthropology at the Centre for Medical Science and Technology Studies, Department of Public Health, the University of Copenhagen. Her research explores the ethical and existential dimensions of medical science and technology. She takes a particular interest in how life is perceived and administered in the interface between the laboratory, the clinic, and the public.
References
Anneberg, Inger, Mette Vaarst, and Nils Bubandt. 2013. ‘Pigs and Profits: Hybrids of Animals, Technology and Humans in Danish Industrialised Farming’. Social Analysis 21 (4): 542–559. https://doi.org/10.1111/1469-8676.12049.
Attride-Stirling, Jennifer. 2001. ‘Thematic Networks: An Analytic Tool for Qualitative Research’. Qualitative Research 1: 385–405. https://doi.org/10.1177/146879410100100307.
Berger, John. 1990. Why Look at Animals? London: Penguin.
Cooper, Jessie. 2018. ‘Organs and Organisations: Situating Ethics in Organ Donation After Circulatory Death in the UK’. Social Science and Medicine 209, pp. 104–110. https://doi.org/10.1016/j.socscimed.2018.05.042.
Dalle Ave, Anne L., Daniel P. Sulmasy, and James L. Bernat. 2020. ‘The Ethical Obligation of the Dead Donor Rule’. Medicine Health Care and Philosophy 23: 43–50. https://doi.org/10.1007/s11019-019-09904-8.
Dam, Mie S., Sandra M. Juhl, Per T. Sangild, and Mette N. Svendsen. 2017. ‘Feeding Premature Neonates: Kinship and Species in Translational Neonatology’. Social Science & Medicine 179: 129–136. https://doi.org/10.1016/j.socscimed.2017.02.039.
Dam, Mie S., Per T. Sangild, and Mette N. Svendsen. 2018. ‘Translational Neonatology Research: Transformative Encounters Across Species and Disciplines’. History and Philosophy of the Life Sciences 40 (1): 21. https://doi.org/10.1007/s40656-018-0185-2.
Dam, Mie S. and Mette N. Svendsen. 2018. ‘Treating Pigs: Balancing Standardisation and Individual Treatments in Translational Neonatology Research’. BioSocieties 13 (2), 349–367. https://doi.org/10.1057/s41292-017-0071-2.
Davies, Gail. 2012. ‘What is a Humanized Mouse? Remaking the Species and Spaces of Translational Medicine’. Body & Society 18 (3–4): 126–155. https://doi.org/10.1177/1357034X12446378.
Douglas-Jones, Rachel. 2017. ‘“Silent Mentors”: Donation, Education, and Bodies in Taiwan’. Medicine Anthropology Theory 4 (4), 69–98. https://doi.org/10.17157/mat.4.4.454.
Druglitrø, Tone. 2016. ‘Care and Tinkering in the Animal House: Conditioning Monkeys for Poliomyelitis Research and Public Health Work’. Bjørkdahl, Kristian and Tone Druglitrø, eds. Animal Housing and Human-Animal Relations: Politics, Practices and Infrastructures. London and New York: Routledge Animal Studies Series.
Forsberg, Anna, Anne Flodén, Annette Lennerling, Veronika Karlsson, Madeleine Nilsson, and Isabell Fridh. 2014. ‘The Core of After Death Care in Relation to Organ Donation—a Grounded Theory Study’. Intensive and Critical Care Nursing 30 (5): 275–282. https://doi.org/10.1016/j.iccn.2014.06.002.
Fox, Renée. C. and Judith P. Swazey. 2013. Spare Parts: Organ Replacement in American Society. Transaction Publishers.
Friese, Carrie. 2019. ‘Intimate Entanglements in the Animal House: Caring for and about Mice’. The Sociological Review 67 (2): 287–298. https://doi.org/10.1177/0038026119829753.
Friese, Carrie. 2013. ‘Realizing Potential in Translational Medicine: the Uncanny Emergence of Care as Science’. Current Anthropology 54 (S7): S129–S138. https://doi.org/10.1086/670805.
Friese, Carrie and Adele E. Clarke. 2012. ‘Transposing Bodies of Knowledge and Technique: Animal Models at Work in Reproductive Sciences’. Social Studies of Science 42 (1): 31–52. https://doi.org/10.1177/0306312711429995.
Govindrajan, Radhika. 2018. Animal Intimacies: Interspecies Relatedness in India’s Central Himalayas. Chicago: University of Chicago Press.
Haraway, Donna J. 2008. When Species Meet. Minneapolis: University of Minnesota Press.
Hoeyer, Klaus and Anja M. B. Jensen. 2011. ‘Organ Donation and the Ethics of Muddling Through’. Critical Care 15 (1): 109. https://doi.org/10.1186/cc9379.
Hoeyer, Klaus and Anja M. B. Jensen. 2013, ‘Transgressive ethics: Professional work ethics as a perspective on “aggressive organ harvesting”’. Social Studies of Science. 43 (4): 598–618. https://doi.org/10.1177/0306312712460341.
Jensen, Anja M. B. 2009. ‘Mistede Liv og Nye Chancer: Kropsdelenes Komplekse Sociale betydninger i Organdonationsfeltet’. Tidsskrift for Forskning i Sygdom og Samfund. 11: 31–50.
Jensen, Anja M. B. 2011. Orchestrating an Exceptional Death: Donor Family Experiences and Organ Donation in Denmark . PhD series no. 69. Department of Anthropology. University of Copenhagen.
Jensen, Anja M. B. 2011a. ‘Searching for Meaningful Aftermaths: Donor Family Experiences and Expressions in New York and Denmark’. Sites: a Journal of Social Anthropology and Cultural Studies . 8 (1): 129–148.
Jensen, Anja M. B. 2016. ‘“Make Sure Somebody Will Survive from This”: Transformative Practices of Hope Among Danish Organ Donor Families’. Medical Anthropology Quarterly 30 (3): 378–394. https://doi.org/10.1111/maq.12278.
Jensen, Anja M. B. 2017. ‘Guardians of “the Gift”: the Emotional Challenges of Heart and Lung Transplant Professionals in Denmark’. Anthropology & Medicine 24 (1): 111– 126. https://doi.org/10.1080/13648470.2016.1193329.
Jensen, Anja M. B. & Johanne B. Larsen. 2020. ‘The Public Debate on Organ Donation and Presumed Consent in Denmark: Are the Right Issues Being Addressed?' Scandinavian Journal of Public Health. https://doi.org/10.1177/1403494819833797.
Jericho, Barbara G. 2019. ‘Organ Donation After Circulatory Death: Ethical Issues and International Practices’. Anesthesia & Analgesia 128 (2): 280–285. https://doi.org/10.1213/ane.0000000000003448.
Kirk, Robert. 2016. ‘Care in the Cage’. Bjørkdahl, Kristian and Tone Druglitrø, eds. Animal Housing and Human-Animal Relations: Politics, Practices and Infrastructures . London and New York: Routledge Animal Studies Series.
Kleinman, Arthur. 2009. ‘Caregiving: the Odyssey of Becoming More Human’. The Lancet 373 (9660): 292–293. http://dx.doi.org/10.1016/S0140-6736(09)60087-8.
Latimer, Joanna. 2013. ‘Being Alongside: Rethinking Relations amongst Different Kinds’. Theory, Culture & Society 30 (7–8): 77–104. https://doi.org/10.1177/0263276413500078.
Latimer, Joanna and Daniel López Gómez. 2019. ‘Intimate Entanglements: Affects, More-Than-Human Intimacies and the Politics of Relations in Science and Technology’. The Sociological Review 67 (2): 247–263. https://doi.org/10.1177/0038026119831623.
Lock, Margaret. 2002. Twice Dead: Organ Transplantation and the Reinvention of Death. Berkeley: University of California Press.
Lomero, Mar, Dale Gardiner, Elisabeth Coll, Bernadette Haase-Kromwijk, Francesco Procaccio, Franz Immer, Lyalya Gabbasova, Corine Antoine, Janis Jushinskis, Nessa Lynch, Stein Foss, Catarina Bolotinha, Tamar Ashkenazi, Luc Colenbie, Andreas Zuckermann, Miloš Adamec, Jarosław Czerwiński, Sonata Karčiauskaitė, Helena Ström, Marta López-Fraga, and Beatriz Dominguez-Gil. 2019. ‘Donation after Circulatory Death Today: an Updated Overview of the European Landscape’. Transplant International 33 (1): 76–88. https://doi.org/10.1111/tri.13506.
Lynch, Michael E. 1988. ‘Sacrifice and the Transformation of the Animal Body into a Scientific Object: Laboratory Culture and Ritual Practice in the Neurosciences’. Social Studies of Science 18 (2): 265–289. https://doi.org/10.1177/030631288018002004.
Mattingly, Cheryl. 2014. Moral Laboratories: Family Peril and the Struggle for a Good Life. Oakland: University of California Press.
Mol, Annemarie. 2008. The Logic of Care: Health and the Problem of Patient Choice. New York: Routledge.
Mol, Annemarie, Ingunn Moser, and Jeanette Pols, eds. 2010. Care in Practice: On Tinkering in Clinics, Homes and Farms. Bielefeld, Germany: Transcript Verlag
Nelson, Nicole C. 2018. Model Behavior: Animal Experiments, Complexity, and the Genetics of Psychiatric Disorders. Chicago: University of Chicago Press.
Nordfalk, Francisca, Maria Olejaz, Anja M. B. Jensen, Lea Larsen Skovgaard, and Klaus Hoeyer. 2016. ’From Motivation to Acceptability: a Survey of Public Attitudes Towards Organ Donation in Denmark’. Transplantation Research 5 (5): 1–8. https://doi.org/10.1186/s13737-016-0035-2.
Olejaz, Maria. 2017. ‘When the dead teach: Exploring the postvital life of cadavers in Danish dissection labs. Medicine Anthropology Theory 4 (4): 125–149. https://doi.org/10.17157/mat.4.4.310.
Rémy, Catherine. 2009. ‘The Animal Issue in Xenotransplantation: Controversies in France and the United States’. History and Philosophy of the Life Sciences 31 (3–4): 405–428
Rheinberger, Hans-Jorg. 1997. Toward a History of Epistemic Things: Synthesizing Proteins in the Test Tube. Stanford: Stanford University Press.
Sharp, Lesley A. 2006. Strange Harvest: Organ Transplants, Denatured Bodies, and the Transformed Self. Berkeley: University of California Press.
Sharp, Lesley A. 2013. The Transplant Imaginary: Mechanical Hearts, Animal Parts, and Moral Thinking in Highly Experimental Science. Berkeley: University of California Press.
Sharp, Lesley A. 2017. ‘The Moral Lives of Laboratory Monkeys: Television and the Ethics of Care’. Culture, Medicine, and Psychiatry 41: 224–244. https://doi.org/10.1007/s11013-017-9530-2.
Sharp, Lesley A. 2019. Animal Ethos: the Morality of Human-Animal Encounters in Experimental Lab Science. Oakland: University of California Press.
Sharp, Lesley A. 2019a. ‘Interspecies Engagement in Medical Anthropology’. Medical Anthropology Quarterly 33 (1): 163–167. https://doi.org/10.1111/maq.12493.
Svendsen, Mette N. and Lene Koch. 2013. ‘Potentializing the Research Piglet in Experimental Neonatal Research’. Current Anthropology 54 (S7): S118–S128. https://doi.org/10.1086/671060.
Svendsen, Mette. N. 2015. ‘Selective Reproduction: Social and Temporal Imaginaries for Negotiating the Value of Life in Human and Animal Neonates’. Medical Anthropology Quarterly 29 (2): 178–195.
Svendsen, Mette N. 2017. ‘Pigs in Public Health’. Critical Public Health 27 (3): 384–390. https://doi.org/10.1080/09581596.2017.1282155.
Svendsen, Mette N. 2020. ‘Pig-Human Relations in Neonatology: Knowing and Unknowing in a Multi-Species Collaborative’. Seeberg, Jens, Andreas Roepstorff, and Lotte Meinert, eds. Biosocial Worlds. London: UCL Press.
Thompson, Charis. 2013. Good Science: the Ethical Choreography of Stem Cell Research. Cambridge: MIT Press.
Wolf, Cary. 2009. What is Posthumanism? Minneapolis: University of Minnesota.