Although many suffering from congenital heart defects (CHDs) have seen their conditions become chronic in Denmark today, the risk of complications, deteriorations, and further surgical interventions often lurk in the future. Building on fieldwork in outpatient clinics in Denmark and the homes of families living with CHDs, I explore the role outpatient encounters play in families’ efforts to understand and navigate the prognoses of CHDs by examining how they become routine punctuations and images of uncertainty, and how they play into families’ efforts to prepare for futures where CHDs might develop negatively while also trying to keep such scenarios at bay. I argue that these encounters exemplify, generate, and tentatively curb the particular uncertainties of living with CHDs. Hence, I suggest that they can be thought of as prognostic calibrations—a conceptual oxymoron that encapsulates the anxiety and uncertainty that I show persist around CHD prognoses despite many efforts by families and healthcare staff to establish routine, a sense of security, and certainty.
Prognostic Calibrations
Throughout outpatient encounters for families living with congenital heart defects in Denmark
—
Abstract
Introduction
Four-year-old Martin has come into the outpatient clinic for his yearly check-up with his parents, Louise and Allan. [1] Although Martin is not currently affected by his moderate CHD, the surgery he underwent at the age of one might not last forever—a leaky valve could at some point create problems. As Martin enthusiastically plays along when nurse Kristiansen uses animals on the wall to measure his height and performs an ECG reading during a quiet game, he seems completely unperturbed. Dr Madsen puts on a cartoon for him. [2] Louise and Allan, however, want Martin to pay attention to the scan, as he’d been worrying that the doctor would have to cut him open to see his heart.
The lights are dimmed, and the room turns silent and tense. Images of Martin’s heart light up the room. ‘Those images, they look like a big mess,’ Allan had previously told me about the grainy images that constantly move and change shape as the heart beats and the cardiologist moves the transducer around on Martin’s chest. After a long period of silence interrupted only by the faint clicking of screenshots and the swooshing sound of blood flowing, Dr Madsen proclaims, ‘Martin, your heart looks amazing—it is working just like it should,’ adding to Allan and Louise that the valve is still leaky but has not deteriorated. They want to know more, so Dr Madsen explains that the valve is working as well as it can, given Martin’s heart disease.
‘Disease?’ Allan asks, his voice raised.
Seemingly surprised at the outburst, Dr Madsen replies, ‘Or heart defect,’ and emphasises that Martin does not need any special consideration or restrictions, not even the extra dental visits his parents thought were obligatory. All his teeth need is thorough brushing to avoid infections, which could travel to the heart.
As we leave, Allan explains to me that he needed to know if they were ‘heading in a completely different direction’. They see Martin’s CHD as ‘fixed’, whereas heart disease, to them, is something that persists. Check-ups, however, remind them that this might not be the case.
Like Martin, around sixteen thousand children and young people under the age of 18 live with a CHD in Denmark (Hjerteforeningen n.d.). Worldwide, CHDs are the most common major birth defects (Linde et al. 2011, 2241). When open-heart surgery for CHDs was introduced in Denmark in the 1960s, risks were high, outcomes often dismal, and many struggled to live with untreated CHDs or simply died because surgery was only possible for older children of a certain weight (and even this was not guaranteed) (Jacobsen et al. 2010, 39; Sundhedsstyrelsen 2016, 4). Today, immense advances in diagnostics and treatment in Denmark (and other countries with healthcare systems geared for high-quality treatment) have turned many CHDs into chronic conditions (Jacobsen et al. 2010, 40; Lüscher 2017, 2021). In Denmark, treatment for CHDs is of high quality (Jenkins 2017, 2733; Sundhedsstyrelsen 2016, 5, 9) and is free for all through a tax-financed rather than insurance-based healthcare system, such as that of the United States. A recent study reported a 100% 30-day survival rate and 93% 10-year survival rate for children in Denmark who had undergone surgical or catheter-based treatment for a CHD between 2003 and 2015 (Larsen et al. 2017, 2725). However, as children with CHDs are living longer, it is becoming increasingly clear that long-term survival is still reduced for patients who have undergone surgical or catheter-based treatment (compared to the background population) and that living with surgically treated CHDs can entail living, for instance, with reduced exercise capacity as well as risks of complications, deteriorations, and further surgical interventions (Bouma and Mulder 2017, 909, 917; Larsen et al. 2017, 2727, 2731; Lüscher 2017, 2021).
As ‘follow-up programs—within the logic of epidemiology and biomedicine—make sense as a way of controlling the imminent clinical uncertainty that lingers’ (Løvschal-Nielsen, Andersen, and Meinert 2017, 101), lifelong medical check-ups have become an integral and inevitable part of life for many children with CHDs. However, as Martin’s story epitomises, check-ups do not just have a biomedical function; they also have implications for families’ lives and their understandings of CHDs, particularly concerning prognoses. They generate questions such as: will there be more heart surgeries? When? How? Could the surgeries fail? Will I/my child live a shorter life? Will that life be severely limited?
Even in cases like Martin’s, where, so far, there have not been significant complications or daily limitations, check-ups still punctuate everyday life and generate anxiety about the future. So, although hospitalisation and heart surgeries are crucial, dramatic, and often traumatic moments, check-ups are the most recurrent clinical intervention and reminder of CHDs that shape life for families in important ways in the many months and years that follow surgery. Still, we have yet to see contemporary qualitative research focused on outpatient encounters. Acknowledging the critical and complex role they play is crucial because, as Dr Knudsen put it during an interview:
Down here [in the outpatient clinic] many have forgotten us, and that’s the coolest thing! They have been at home for maybe two years without seeing us, and then the letter arrives where they are reminded that there is something [nervous laugh]. And then typically they come [in] very anxious. Many, really many, are clearly anxious, and the children are also very much on edge. Then you have to acknowledge that to them it’s a big thing. For me, it’s a small thing. Because up on the ward [where children are admitted for heart surgery], everyone knows that it’s a big thing.
This article explores how check-ups are ‘a big thing’ by looking at the role outpatient encounters play in families’ efforts to understand and navigate the prognoses of CHDs. I show how outpatient encounters become routine punctuations, engender images of uncertainty, and play into the families’ efforts to simultaneously prepare for futures with CHDs while keeping their negative potential at bay. I show that outpatient encounters are events where the particular uncertainties of living with CHDs are both exemplified, generated, and tentatively curbed. As such, I suggest that it can be helpful to look at outpatient encounters as prognostic calibrations, understood as families’ continuous and often fraught attempts to come to know, adjust to, and reconcile biomedical prognoses. Prognostication involves ‘a medical judgment about the likely or expected development of a disease’ (Cambridge English Dictionary n.d.b], whereas to calibrate is ‘to make, adjust, or check the setting (= the position) of the controls used to make measurements with a tool or measuring device’ (Cambridge English Dictionary n.d.a). Prognostic calibrations are adjustments to understandings, knowledge, hopes, and fears rather than to an instrument. Nevertheless, they build on medical technology, which is perhaps why families hope for somewhat precise estimations of the future. Together they form an oxymoron because calibrations (as adjustments to exact measuring points) in this context can never be fully realised (or at least are very difficult to realise) due to the fact that prognoses are predictions and therefore inherently uncertain. Indeed, the very reason for continuing check-ups is uncertainty regarding prognoses. Prognostic calibrations, therefore, help to highlight the anxiety, high stakes, and uncertainties that I will show persist despite continuous attempts to establish routine, a sense of security, and certainty.
Addressing the prognoses of chronic conditions in outpatient encounters
Uncertainty is an enduring topic in medical anthropology (Taussig, Hoeyer, and Helmreich 2013, S9) because ‘sickness in particular, and crisis in general, pose questions about our very sense of existence and non-existence’ (Jenkins, Jessen, and Steffen 2005, 9). For some patients and their families, the biomedical prognosis is critical in navigating such uncertainty (e.g., Christakis 1999, 31–32), while for others the fear of where such navigation will lead makes uncertainty, or paying little attention to prognosis, a preferable option (e.g., Whyte 2002; Bluebond-Langner 1996). Either way, previous anthropological scholars working on risk and control in life with illness have emphasised how ‘in matters of life and death, knowledge is usually only a little, no matter how much we might like it to be otherwise and despite the undoubted power of western biomedicine’ (Jenkins, Jessen, and Steffen 2005, 24). With regard to congenital conditions, scholars have shown how the emergence and routinisation of new biomedical technologies create new dilemmas and uncertainties related to risk—both for foetuses not yet diagnosed and for adults living with (and despite) previous dismal prognoses (Maynard 2006; Rapp 2000). Despite the shortcomings and complexities of prognoses, the stakes are often high. For patients and their families, the stakes concern tensions and balances between hope and despair; assumptions of or actual differing needs for knowledge within families; potentially grave misunderstandings of biomedical language and statistics; and difficult decisions concerning the planning of daily life, care, treatment, and, for some, death (Russ & Kaufman 2006, 105; Christakis 1999, 31). For doctors, the stakes concern their ability to treat and monitor conditions; their relationships to patients; and their own professional authority, reputations, and accountability (Christakis 1999, 33, 63, 64).
Within sociology and anthropology, scholars have, among other things, examined how much effort health professionals, patients, and their relatives put into creating hope despite dire prognoses (Mattingly 2010) and the ways in which statistical prognoses both create and take away possible futures (Jain 2007). Additionally, such scholars have explored the ways in which prognoses are often withheld from or only subtly hinted at to patients, especially children (Bluebond-Langner 1980; Christakis 1999; Timmermans and Stivers 2018); the ways in which (chronic) prognoses instil anxiety and doubt while also motivating self-care (Wolf-Meyer and Callahan-Kapoor 2017); and the ways in which prognoses and interpretations of risk (or, often, the lack thereof) influence the use of new biomedical technologies (Maynard 2006; Shim, Russ, and Kaufman 2007). Despite this variety, Stefan Timmermans and Tanya Stivers have pointed out that ‘most of the literature on prognosis deals with end-of-life discussions, even though prognosis also matters greatly for chronic conditions’ (2018, 13). Fonseca, Fleischer, and Rui have also argued that there is an ‘epistemic uncertainty that surrounds chronic illness’ because ‘if the condition were thoroughly understood (so our reasoning goes), research would produce a cure, or at least a more efficient manner to predict and manage negative aspects of the condition, and so, it would no longer be considered chronic’ (2016, 595). In this sense, this article is also a contribution to the understanding of how important prognosis is for those living with chronic illness. Even though death may not be waiting just around the corner from the check-up, patients with chronic conditions and their families still ‘wonder what their future holds’ (Timmermans and Stivers 2018, 14). This is especially pertinent when it comes to those suffering from CHDs, as medical prognostication for individual patients is challenging due to previous and continuous advances in treatment, the rarity of some CHDs, and sometimes vast differences in severity within the same diagnoses (Reid et al. 2006, 354; Vonder Muhll, Cumming, and Gatzoulis 2003, 1598–99).
‘Hospitals are places of intensity, of life-and-death drama’ (Long, Hunter, and Van Der Geest 2008, 71, 72), and are, therefore, also the sites of a growing body of ethnographies. Curiously, and wrongfully I would argue, it seems like such intensities and dramas have been considered more present or worthy of examination in ward rather than outpatient encounters. Similarly, hospitalisation for surgical intervention has been the focus of most contemporary qualitative research on clinical encounters for patients with CHDs (e.g., Obas et al. 2016; Re, Dean, and Menahem 2013; Thomi, Pfammatter, and Spichiger 2019; Ytting and Thing 2013). The few studies that have briefly mentioned outpatient encounters have indicated that they are unwelcome reminders of difference, that continuity in relations with staff is important, that children and adolescents with CHDs rely on parents and rarely ask questions of healthcare professionals, and also (tellingly) that parents find it difficult to ask questions (Knowles et al. 2016, 14–17; Kendall et al. 2003a, 23; Kendall et al. 2003b, 15–16; Rosenwein et al. 2019, 44–47). In a study of parental consent for paediatric heart surgery more than three decades ago, sociologist Priscilla Alderson did observations on and interviews about outpatient encounters (1988). She argued that ‘masses of information […] in the limited time of the clinic, like icebergs, remain largely submerged’, just as one of the mothers in her study emphasised how ‘knowing the future was a matter of seeing visit by visit’ (1988, 130). It is precisely this way of coming to see the future, outpatient visit by visit, that I explore in this article. I therefore delve into the efforts that families living with CHDs make to come to know, understand, and handle prognoses—not only as a matter of life expectancy, but also in terms of possible surgeries, deteriorations, and limitations.
The study
This article builds on several ethnographic fieldwork stays during 2016 in the only three paediatric cardiology outpatient clinics in Denmark, all of which are situated in public university hospitals and of which two were performing surgery on patients suffering from CHDs at the time. In the clinics, I observed 87 outpatient encounters. [3] The analysis also draws on semi-structured interviews conducted between the spring of 2016 and the spring of 2018 with 38 members of 16 different families (22 parents, eight children and young people with CHDs, seven siblings, and one grandparent). [4] Ten families were interviewed in their homes, seven of which I met several times, including the four families in focus here. Furthermore, I did semi-structured interviews with four nurses and four cardiologists from the outpatient clinics and two wards. As my project was about living with CHDs in a broad sense, I also observed ward encounters and attended patient association events organised by the Danish Heart Foundation. [5]
I have focused on children and young people, aged 0–18, suffering from a diverse range of CHDs. All had undergone or were just about to undergo surgery for a CHD and were still going for check-ups at a paediatric cardiology clinic. Some had intervals lasting just a few months, while others only had to get checked once a year. The children/young people with CHDs in the seven families whose members made up my key informants had moderate or complex CHDs, which meant they would need specialised medical care and continuous check-ups throughout their lives (Warnes et al. 2008, e154–55).
Observing over 80 consultations allowed me to see the subtle intensities, dramas, and complexities of these outpatient encounters for the families involved. Meeting families in their homes as they went about their everyday lives outside the clinic added layers of meaning to my experience of the high stakes of outpatient encounters. Overall, my fieldwork allowed me to witness the extremes of life with CHDs, from dramatic hospitalisations to the everyday lives in which families were often able to background CHDs to some extent, helping me realise the meaning of check-ups as events where these extremes both collide and are connected.
Routine punctuations
Sixteen-year-old Mads is starting efterskole [a Danish type of boarding school] and Dr Jensen is very interested in his new adventure. Mads explains that his track is focused on science.
‘Then there’ll probably be more chess playing than smoking weed,’ Dr Jensen teases.
Mads laughs. At the edge of the examination room, hugging a jacket, his father Hans also lets out a few muffled laughs. Dr Jensen explains to me that, apart from his moderate CHD, Mads also had an acute childhood illness that causes inflammation of the blood vessels and can weaken the coronary artery walls.
‘We always come up with something new when you come here,’ Dr Jensen jokes.
‘Yes,’ Nurse Andersen adds, ‘it really is quite astonishing that you still want to come here!’
Mads, however, seems relaxed and, without hesitation, takes off his shirt, revealing a long scar running down the middle of his chest and a pacemaker under the skin on the left side. Dr Jensen starts scanning, and Mads follows his heart on the screen.
‘It looks really good,’ the doctor concludes, and Mads gives his dad a relieved look.
When I visit Mads and his family in their home a few months later, he tells me that the joking atmosphere is important, as it ‘keeps one’s spirits up’. Although coming to the outpatient clinic every six months ‘has just become routine’, Mads is still nervous and hopes that ‘nothing is wrong’. As Hans also has a CHD and collapsed in his twenties, the family fear that Mads might also collapse. His thirteen-year-old sister Diana tells me how she is sometimes ‘afraid that his pacemaker will suddenly stop working. That is, that he cannot live any longer’. This fear is not present every day, his mother Gitte explains, but ‘it is when he is in for check-ups, and we turn it over in our minds’. Hans and Mads’s coordinated check-ups and their tradition of going to a restaurant afterwards help alleviate some of Mads’s anxiety and, in his words, they ‘always have a good time with it’. Nevertheless, he feels that ‘you get sicker from being in there’. The waiting room is especially troubling for him, as ‘a lot of people come in that are really, really unwell. That’s not so nice when you’re actually okay yourself’.
Mads’s experience of feeling sick when encountering visibly sick bodies in the clinic is something of an exception, as most children and young people with CHDs do not look sick at first sight (Svensson, Wahlberg, and Gislason 2020, 127–129). However, he ended up in the adult waiting room down the hall during his last visits, and ‘80 % of those who are here, they are old people who look like they’re dying. That gives you the psychological [feeling] of getting a little worse’, he explained. Nevertheless, his experience highlights how the outpatient encounter creates a sort of bodily ‘dys-appearance’, which, Drew Leder suggests, happens when the body comes into explicit awareness ‘in a dys state’ (1990, 84, 86; italics in original). This dys-appearance draws attention to children and young people’s bodies as dysfunctional now but also potentially more dysfunctional in the future, thus presenting them as being continuously ‘open for repair’ (McLaughlin and Coleman-Fountain 2014, 76). This is illustrated by Mads quite literally seeing the future he fears in the waiting room or while awaiting the doctor’s verdict on his heart scan. Bodies are also compared to those of healthy peers through doctors’ questions about physical capabilities, such as, ‘How well can you keep up with your classmates in physical education?’ Sometimes check-ups force children and young people to feel the dysfunction through a cardiac stress test, often just referred to as a ‘cycle test’. Sara, 17 years old and with a complex CHD, described how ‘when you have to do the big cycle-test, then you can get the feeling that “oops, there really is something wrong”’.
Although Leder’s phenomenological framework refers to situations involving a sudden loss of bodily function (which are rarely encountered by people suffering from CHDs), he points out that, ‘Dys-appearance can also arise in a technical context, as when I am subjected to a doctor’s physical exam. My body becomes a collection of organs, a mass to be studied and palpated’ (1990, 98). Similarly, Nurse Andersen described how ‘most heart-children do not notice their heart in their daily lives—most live a fairly normal life’. She went on to add that ‘there are also many children who get scared when they go to the doctor to get examined’. To alleviate such reactions and ease examination of and communication with the children and young people with CHDs, doctors and nurses do their best to provide distractions in the shape of cartoons, soap bubbles, toys, or packets of raisins for the younger children, whereas young people like Mads are often distracted with jokes and lively talk. Despite these efforts, I found that outpatient encounters often remain what Knowles et al. have called ‘critical flashpoints’ (2016, 14)—that is, prominent and unwanted reminders of CHDs that disturb patients and their families’ efforts to live as normal lives as possible (Rosenwein et al. 2019, 45; Svensson, Wahlberg, and Gislason 2020, 126). Sara, for example, described how simply ‘stepping in, around once a year, into the hospital is kind of weird, because you feel completely rask [healthy/recovered]’— even though she had a complex CHD that reduced her physical stamina, required medicine, and would entail more surgical treatment.
Dr Knudsen argued that ‘there are those who are not anxious at all; they have been here six thousand times […] It is typically those with very long check-up intervals who are reminded in an uncomfortable way’. Although I agree with Dr Knudsen (and with the similar findings described in Knowles et al. 2016, 7) that check-ups at longer intervals may punctuate the everyday lives of families living with stable CHDs more intensely, I found anxiety and discomfort to be present in all the families I followed, even those who experienced more routine and seemingly cheerful and/or relaxed outpatient encounters (like Mads’s, for example). Nanna, mother of 12-year-old John, who had a complex CHD, also described them as routine, though this did not remove all worry:
Often there is nothing. It is just to see where we’re at. We try to make it a bit cosy, and the day we go in to get the heart checked, he takes the day off from school. He has been coming there for many years, so he has his own routines […] It’s actually not very present as such. It is when we have a feeling that something is wrong or something like that […] You are always a little nervous when you look at that heart-thing, that scanner.
Check-ups follow a specific pattern in accordance with biomedical guidelines and practices. However, it is not only the repetition and familiarity of these patterns that create routines. Many families have traditions that aim to make check-ups pleasant and recognisable, such as going to a restaurant afterwards (like Mads), taking the day off from day-care or school, going on shopping trips, watching the same cartoon during scans, racing the doctor down the hallway, or getting an ice-cream as a reward. However, in Nurse Andersen’s experience, neither routine nor traditions provide anything more than a thin veneer of protection from anxiety and discomfort, as ‘things should be the same every time—also the same doctor saying the same things. It should have the same pattern. That gives comfort. We experience that, when things are not normal […] then, even though they have been coming here for many years, their world collapses’.
The same doctor, the same pattern, the same words—together with routine, traditions, and distractions—help to alleviate, without ever completely removing, the interruption and unwelcome reminders that check-ups entail. These routine punctuations of life thus seem to be short but intense experiences for most families, where recognisability and fun do not preclude feeling the discomfort associated with attention being drawn to the body’s dysfunction and the uncertain outcomes of examinations. Much of this anxiety is caused by uncertainty about the prognosis, which, as we will see, check-ups, if ever, rarely eliminate.
Images of uncertainty
‘You usually take 22 images; now you have taken 31,’ nine-year-old Fanny says to Dr Sørensen.
‘Have you been keeping an eye on that?’ Maria asks her daughter, seemingly surprised, although she and her husband, Lars, have been watching both Fanny and the monitor intensely. Fanny comes into the clinic every second month to get checked for her moderate CHD, as she is currently congesting fluid. Dr Sørensen concludes that a cardiac catheterisation needs to be booked to measure the pressure inside the heart and asks if Fanny wants to hear about the procedure. [6]
Maria suggests that Fanny could go outside and talk to somebody, but Fanny seems puzzled: ‘Who?’ Dr Sørensen emphasises that she is welcome to stay, and Fanny replies, ‘I’m staying!’ Although she has undergone two catheterisations, Fanny has no memory of them. Maria points to her groin where the catheter was inserted, and Fanny exclaims, ‘I still have the scars from that!’
A few months later, Maria writes to me that the catheterisation was cancelled, which ‘leaves us with unresolved questions a little longer’. She has had to go on sick leave because ‘this cancellation just hit me hard emotionally’.
Two days later, in the clinic waiting room, Maria tells me that cancellations are difficult because of the energy it takes to prepare for examinations and especially the surgeries they may result in. Last year’s one-month hospitalisation reminded them that ‘there were some that didn’t make it home’, she whispers, to avoid Fanny overhearing. Today, Dr Sørensen changes Fanny’s medication and talks about the catheterisation, which now has a new date. Fanny just wants to ‘get it over with’, but says pleadingly, ‘I hope they don’t take me in [for surgery] again on 21 April.’ This year she wants to celebrate her birthday at home.
A month later, in their home, Lars and Maria tell me how the doctors have gone back and forth between different treatment options, involving multiple cancellations, postponements, and new surgical plans. ‘It’s all so uncertain—it’s so unpredictable how it will turn out! They say, “Now it’s time,” and then it wasn’t now anyway,’ Maria concludes, sighing deeply. Thanks to a new 3D printing technology that has allowed for the creation of a model of Fanny’s heart and a new surgeon with a different approach, most of Fanny’s CHD was corrected a year ago. One of the remedial repairs, however, has not been undone.
A week ago, the doctors doing the catheterisation suggested that it might be causing the congestion of fluids. This might mean another ‘big surgery’, Lars explains. ‘I think they won’t do it until the fall,’ he speculates, only to admit despairingly that ‘it’s just guessing again’.
In Fanny’s case, three heart surgeries and three cardiac catheterisations in just nine years, and more surgeries looming, are the cruces of the uncertainty of living with CHD. For others, like Martin and his family in the opening vignette, it is the other way around—few surgeries have allowed a stable and relatively normal life, but the possibility of deterioration and new surgeries, however far away or unlikely, create their uncertainties. So, despite variations in circumstances, stakes, and scales of intrusion, uncertainty persists for all the families I observed. For several reasons, check-ups are particularly important and intense sites for dealing with such uncertainties, not just in the shape of doctors that ‘calibrate parents’ expectations of what is likely going to happen’ (Timmermans and Stivers 2018, 18), but also in the shape of families’ efforts to calibrate the prognosis.
First, although children and young people with CHDs can experience symptoms such as congestion of fluid (like Fanny), breathlessness, fatigue, pain, or fainting fits when CHDs deteriorate or older surgical solutions wear out, symptoms are not always easily identifiable for patients/families, as they can develop over long periods or be confused with natural changes. As Dr Poulsen said, ‘It could also just be that you get tired after starting day-care.’ In other cases, ‘you do not actually think that they are doing as badly as they actually are because there is nothing to see on them’, as Mette, mother of four-month-old Matthias with a moderate CHD, told me. She and her husband experienced a particularly ‘shitty scan’ which showed them that Matthias’s CHD had deteriorated without them noticing.
Secondly, as diagnosis and the first round(s) of surgeries for CHDs often happen in the first few years of life (Larsen et al. 2017, 2726–2727; Olsen 2010, 3), many children and young people with CHDs are missing at least some of their illness story (Kendall et al. 2003b, 13), and not least the prognostic conversations that occur around these events. For them, outpatient encounters are therefore particular sites of tension, confusion, as well as information. As they undergo particularly turbulent periods, like Fanny, or get older and approach adult care, outpatient encounters and their images become increasingly important because they help them understand ‘what I am in for’, as 17-year-old Sara phrased it.
Thirdly, outpatient encounters are intense sites for the calibration of prognoses because the stakes are so high. In a study of everyday life and coping among children with juvenile arthritis, Cornelia Guell found that, regardless of frequency, outpatient encounters were ‘major landmarks’ for the children because ‘they can lead to either an increase or a decrease in the dosage of their main medication and thus more or fewer bitter pills to swallow’ (2007, 888). In comparison, outpatient encounters for patients with CHDs can result not only in changes to medicines, but also in rather dramatic and sometimes risky modifications to the body, where the chest is cut open, the chest bone sawed through, the heart stopped temporarily, and surgeons cut into the organ that symbolises life, personhood, love, and emotions (Røpcke 2016, 24, 60). In Fanny’s case, Maria worried about ‘what it means for such a small body to have to be opened up again, now for the fourth time’, while Lars emphasised that ‘it’s also the psychological [impact] for Fanny!’ Fanny herself felt that ‘it’s rather crazy that it is possible to open up and sew inside the heart’ and admitted that especially surgeries made her ‘wish I didn’t have a different [i.e., defective] heart’. The high stakes also make communication a delicate balance because worst-case scenarios, however unlikely, are always quite extreme. As Dr Petersen explained, ‘The valve can get narrowed, then you have to have surgery. You can get rhythm disturbances, and then you can die. The operation can go wrong, and then you can die. Everything can go wrong, and you can die. They hear that.’
Finally, prognostic calibrations take place particularly during outpatient encounters because the focus of many consultations is the visualisation of otherwise hidden heart defects through ultrasound images of the heart (echocardiography) (see Image 1). [7]
According to Irene, mother of five-year-old Karl, who has a complex CHD, this means that they are ‘a very technical check-up, where they [doctors] use a lot of their resources on looking at these scanning images and focus on how it looks and stuff. And I cannot follow all that scanning’. Previous scholars have emphasised how powerful biomedical imaging technologies (or the lack thereof) can be in shaping understandings of the foetus or body as well as the evaluation of medical care (e.g., Reventlow 2006; Street 2014; Taylor 2008). In the case of CHDs, biomedical monitoring has historically moved from a non-invasive manual technology based on sound in the shape of auscultation to the invasive imaging technologies of heart catheterisations, and from the 1970s the non-invasive imaging technology of echocardiography developed into the cornerstone (Jacobsen 2010, 39). Echocardiography does not detract attention through invasive procedures and concomitant pain and, as sociologist Mildred Blaxter points out, very importantly offers the family a view of the body’s interior produced before their eyes instead of static images of bodily functions to be viewed only by healthcare professionals (2009, 762, 764). Thus, even though scans were often what Allan earlier called a ‘big mess’ for families, having a beating heart rendered visible in real-time, followed by a more or less instantaneous biomedical interpretation, set the stage for meticulous viewing and worrying. I often experienced a mood during scanning that was similar to that noted by Tine Gammeltoft in her descriptions of obstetrical ultrasounds for foetal abnormalities in Vietnam, where ‘it was weirdly quiet, and the atmosphere was tense, everyone’s attention apparently directed toward the scan that was going on’ (2013, S161).
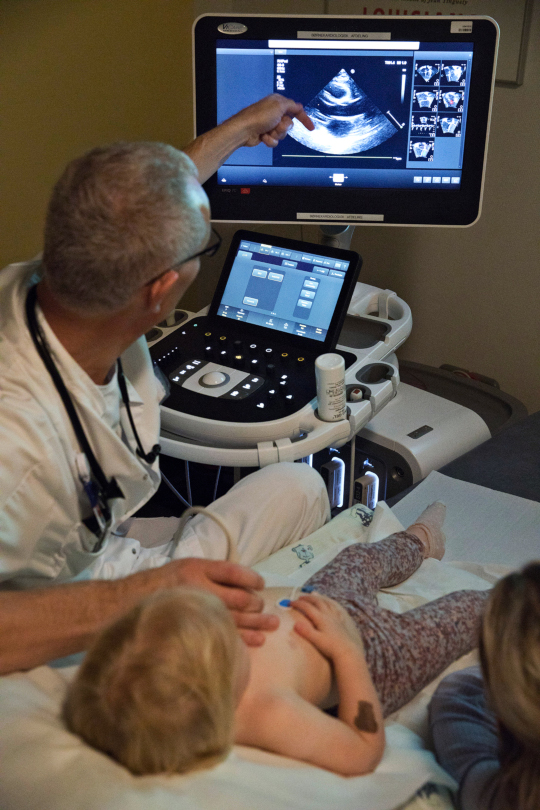
Figure 1. Echocardiography in an outpatient clinic. Photo by Sine Fiig for the Danish Heart Foundation for the book Medfødte Hjertefejl from 2017. (Permission for reuse given by the photographer).
Each of these four factors contributes to the shaping of outpatient encounters as particularly crucial and intense sites for dealing with uncertainty. Yet, although such efforts can create temporary feelings of relief, they far from always create certainty—as Fanny’s story also illustrates. Nanna, mother of John, who has a moderate CHD, also described difficulties with trying to calibrate the future with numbers from heart scans:
I do keep an eye on the pressure in the healthy blood vessel, and then I know roughly where we’re at. […] Are there many years still [to the next surgery]? And yet, you cannot really say anything about it. The pressure of a normal heart is around 20, and when John has had surgery, it has been around 100. […] He might have been in [the clinic] three months ago, when it was still 70, and then suddenly it rose to 100 within three months. So, you can’t really count on it.
With at least 18 types of CHD (all of which can occur in multiple combinations [American Heart Association 2018]), illness trajectories that can differ substantially, some surgical solutions that lack long-term prognoses, and continuous biomedical advances that might (re)create the future, there is no ‘crystal ball’, as Nurse Rasmussen phrased it. It is little wonder then that the prognoses that come out of outpatient encounters often remain with families as images of uncertainty. Previous studies in medical anthropology have also found that ‘the pursuit of reliable predictability is in itself a definitely uncertain project’, and it is therefore often a matter of attempting to control uncertainty rather than succeeding (Jenkins, Jessen, and Steffen 2005, 21). However, Nanna, like Fanny and her family and many others, carried on with their attempts to calibrate prognoses by noticing particular numbers; counting screenshots; or simply paying attention to doctors’ and nurses’ subtle gestures, pauses, silences, or word choices during outpatient encounters.
Preparing for heart futures kept at bay
Dr Christensen, a new doctor to five-year-old Karl and parents Irene and Adam, instructs Karl to lie differently than he usually does during a scan, which also takes a remarkably long time. Knowing how complex Karl’s CHD is, I start to worry about the results. However, after 20 minutes of silence, Dr Christensen concludes that everything looks fine. Irene asks whether Karl’s heart isn’t enlarged.
‘No, it looks fine,’ the doctor replies.
Irene keeps at it: ‘Fine for someone like Karl, or in general?’
Dr Christensen mumbles something and reassures her that it looks fine. After some blood tests, we head for the hospital cafeteria so Karl can get an ice-cream—a family tradition after check-ups (to ‘finish it in a good way’, as Adam later explains to me). Irene points out that they have kept the tradition small in scale ‘because we have never known “how often are we going to come here?”’
When I talk to Irene and Adam four months later, they tell me they were disappointed that it was not the usual doctor examining Karl because a shared history eases communication and their trust in the medical assessment. However, they have chosen to put the issue of Karl’s enlarged heart on hold. Irene acknowledges that such questions are tricky for doctors because ‘enlarged compared to what?’
‘It’s bigger,’ Adam comments.
Irene agrees: ‘It’s bigger, but is it bigger, bigger?’ She concludes that ‘if there was something, the doctor would have said so’.
In subtle ways, questioning the doctor’s assessment of Karl’s enlarged heart and finishing the visit with an ice-cream can be seen as efforts to calibrate and prepare for the possible futures with CHD that are brought into focus during outpatient encounters. However, as is the case with so many other families, if no major changes are found or interventions deemed necessary, the family turn (back) to the strategy of trying to keep futures with CHDs at bay as they return home and immerse themselves in their busy daily routines. In Karl’s case, this strategy entailed trusting the doctor’s judgment that his heart was not yet ‘bigger, bigger’, which would require a heart transplant. At home, Irene and Adam refrained from talking much about Karl’s CHD and especially how it might develop in the future, and tried their best not to be overly cautious nor restrict his dreams for the future, regardless of how unrealistic his current dream of becoming a farmer seemed. Such strategies were possible because Karl, for now, was doing well despite his complex CHD. His sisters, 14-year-old Emma and nine-year-old Mathilde, told me that there was no need to talk much about Karl’s CHD because, as Mathilde said, ‘he is running around playing like a normal child’.
The future is not only fraught with ‘negative uncertainty’, but also ‘positive uncertainty’ (Timmermans and Stivers 2018, 18), such as hopes for more medical advances, which parents especially use to keep negative uncertainty at bay. Adam told me that he felt that ‘time is working really well for us’ in the sense that ‘research is being done on many things’. Such hopes were often built on the families’ own experiences of biomedical advances. For example, a few decades ago surgical solutions for Karl would have been very poor, while it was a new 3D printing technique that enabled Fanny’s CHD to be corrected, something considered impossible during the first years of her life.
Karl’s CHD is on the more complex end of the continuum. However, with regard to check-ups, the movement back and forth between preparing for futures where CHDs might develop negatively and keeping such futures at bay was something I experienced in families across diagnoses, albeit in different ways and on different scales. This seems similar to the ‘compartmentalization’ described by Myra Bluebond-Langner (1996) in her study from the mid-1980s on how families, and in particular siblings and parents, managed the burden of cystic fibrosis (CF) in the United States. She argued that, after the bombardment of and intensive search for information around the time of diagnosis, ‘information is processed and sorted in such a way that particular kinds of information are kept from immediate awareness’—particularly information related to prognosis (idem, 147–48). All this to ‘contain the intrusion’ of illness as much and for as long as possible (1996, 13). Like many colleagues, Dr Knudsen supported and advocated such an approach:
I am always worried about being too negative because it keeps some kids in a self-image that I don’t think is good—as sick. That they cannot and should not and will not, and what I basically mean—despite that you’re sick and that you can drop dead tomorrow—my life philosophy is that at least you should feel well until tomorrow. […] I try to be honest, but I try to turn even the very sick children round.
Often nurses and doctors try to make life ‘until tomorrow’—whether in the shape of death or, more likely, deterioration, the next surgery, or simply just the next check-up—as good and as little affected by the CHD as possible. They do this by emphasising that many children and young people with CHDs should be treated as ‘normal children’, free from restrictions or the intense monitoring of symptoms. This, for example, was the case for Mette, who told me that after her son Matthias had surgery, ‘they have really emphasised to us that we have a completely normal child’, even though Matthias would need further surgery and would have to attend lifelong check-ups. So rather than the biomedical prognoses of a chronic condition and its uncertainties (negative as well as positive) giving cause for altered behaviour and intense practices of self-care, what Matthew Wolf-Meyer and Celina Callahan-Kapoor called ‘chronic subjunctivity’ in their study of patients with type 2 diabetes and patients with insomnia in the US (2017), health professionals prescribe that families of CHD patients should not let the uncertainty of prognosis affect their current lives.
However, as Bluebond-Langner noted about the compartmentalisation of prognoses of CF (1996, 147–56), keeping the future at bay becomes especially challenging, if not impossible, during periods of instability (as in Fanny’s case). Furthermore, it is especially challenging for parents, as ‘the “risk of my child dying of this” has to be their number one [worry]’. Dr Petersen went on to explain that ‘they have been through, many of them, a situation where it has been pretty much a matter of life and death’. Yet, in the many outpatient encounters I observed, such concerns were rarely openly discussed in front of or with the children and young people in question. Dr Knudsen told me, ‘I try not to use the word death,’ just as Maria only dared to whisper the words to me when Fanny was sitting a few seats away. Likewise, when Irene asked about Karl’s enlarged heart, she was asking subtly about his possibly limited life span and how close he was to needing the risky procedure of a heart transplant. After the check-up, Irene explained to me that:
All that about not knowing how his disease develops, that is not something we talk to him about yet. He should just go to school, and now he is starting first grade in the summer, and then he is going to second grade, and then it will be Shrovetide again [a carnival-like festivity for children].
As was the case with most other families, this did not mean that Karl’s parents had not prepared him for possible examinations or surgical interventions in the near future. He told me, for example, that soon ‘they will cut me up somehow’, as his pacemaker would need a change of battery. Rather, it was specifically the fear or risk of death that healthcare staff and parents struggled to figure out how and when children and young people with CHDs should be involved in prognostic conversations, which had often started at a time they did not remember. Given the need for lifelong check-ups, the risk of more open-heart surgeries or deterioration, and the fact that the heart is intimately tied to life and death, parents and healthcare staff are well aware that these conversations are inevitable. For example, despite Maria’s whispering and her attempt to send Fanny out of the consultation room, Fanny still came home one day terrified and asking about death. One of her classmates had suggested that her heart must have been stopped during her surgery and that she had therefore been dead. Her parents then had to carefully explain the procedure of open-heart surgery and the use of a heart-lung machine. However, how and when such conversations should take place are difficult questions. This is partly due to age, but also because far from all CHDs involve a high risk of death, and because many CHD prognoses are very uncertain. As Adam said:
You can easily—what should you say—almost do more harm than good somehow. Because, what kind of scenarios do you bring forth? And if it’s not even on his mind at all, then I cannot see the purpose as things are right now. However, there will definitely be some questions at some point, when others ask [him], or he sees something.
Rather than maintaining a kind of ‘mutual pretense’ over an established dismal prognosis (Bluebond-Langner 1980), it seems as if the uncertainty of the prognostic ‘scenarios’ of CHDs might instead create confusion about prognoses across different family members as suggested by the results of two previous questionnaire studies from Canada and Italy. In one study, only 9% of young people/ young adults, aged 16–20, with complex CHDs and 3% with moderate CHDs had ‘realistic’ views of their life expectancy, and most were overly optimistic (Reid et al. 2006, 353). In the other study, only 3.3% of parents had a ‘correct’ understanding of their child’s prognosis (Chessa et al. 2005, 127). [8]
In a sense, outpatient encounters involve both preparing for feared futures with CHD while also trying to keep such scenarios at bay. Løvschall-Nielsen and colleagues poignantly describe how ‘follow-ups can tame chance and circumscribe clinical uncertainty, but at the same time they call attention to probability and risk’ (2017, 101). It seems then that check-ups are challenging and somewhat paradoxical encounters where prognostic calibrations take place not only in relation to biomedical images, measurements, and tests but also to families’ and healthcare professionals’ ideals of how best to live with CHD, as well as the uncertainty that this entails.
Concluding remarks
In the wake of decades of impressive advances in the field of CHD diagnostics and treatment, I have focused on how families together with healthcare staff relate to the promising yet still uncertain futures of life with CHD(s) in outpatient encounters. I have suggested that this is a matter of regular and continuous prognostic calibrations by showing how outpatient encounters become routine punctuations, engender images of uncertainty, and play into families’ efforts both to prepare for a future (more) affected by CHD and keep it at bay. When Louise and Allan try to figure out whether Dr Madsen’s use of the phrase ‘heart disease’ means their son’s CHD has deteriorated, and when Fanny counts scanning images and, like her parents, struggles to figure out if and when a new surgery might be necessary, they are engaging in prognostic calibrations. On the other side of the examination couch, Dr Madsen contributes to these prognostic calibrations when emphasising that, although still leaky, Martin’s valve has not deteriorated, and thus he does not need any special consideration or restrictions. In this way, prognostic calibrations occur at different scales: sometimes they are dramatic and concern the possibility of open-heart surgery, while at other times it is just the need for extra dental visits that need adjusting.
Prognostic calibrations in CHD-focused outpatient encounters are particular and intense endeavours due to the high stakes involved (e.g., open-heart surgeries and the risk or fear of death), the crucial monitoring function they have, the uneven levels of knowledge in many families, and the central role that live images of the heart play in these encounters. I have shown how outpatient encounters should, therefore, be acknowledged as ‘a big thing’ in the lives of families living with CHD in Denmark. However, in this respect, they are most likely not alone. A range of congenital conditions besides CHDs, such as cystic fibrosis and spina bifida, also previously had high early-life mortality rates but have now, in many cases, transformed into chronic conditions (at least among children with access to high-quality treatment) (Manderson, Cartwright, and Hardon 2016, 20–21; Perrin, Anderson, and Van Cleave 2014, 2099). Still, although death may seem to have been conquered, at least for a while, its shadow still lingers for these very literal chronic conditions. Thus, prognostic calibrations in outpatient encounters are most likely a central part of living with them. Beyond congenital illness in childhood, I would also suggest that prognostic calibrations are becoming an important part of an increasing number of patients’ lives. As chronic conditions proliferate worldwide and preventive medicine continues to develop in countries with healthcare systems geared for high-quality diagnostics and treatment, more and more patients and ‘patients in waiting’ (Heinsen, Wahlberg, and Petersen forthcoming) will be followed in outpatient clinics. The specifics of how such calibrations will take place, what stakes they will concern, and the kinds of routine punctuations they entail will probably differ depending on the condition and location in question.
It is, however, important to note that in many places around the world, there are no outpatient encounters nor prognostic calibrations, simply because conditions remain undiagnosed or untreated. In the case of CHDs, 90% of patients globally live in places without access to adequate diagnostics or treatment (Zheleva and Atwood 2017, 16). The kind of ‘chronic care infrastructure’ (Langstrup 2013) available in different settings around the world may also vary substantially—sometimes to the point that check-ups are either entirely absent or their quality or access severely limited (as described, for example, in the case of CHD patients in Honduras [Worthington 2015, 186, 282]). This variance undoubtedly affects how outpatient encounters take place as well as the kinds of prognostic calibrations that unfold within them.
Finally, navigating and adjusting understandings of prognoses is, of course, not limited to outpatient encounters, or for that matter even to clinical encounters; calibrations occur continuously in the everyday lives of the chronically ill and their families (e.g., Bluebond-Langner 1996). Prognostic calibrations are just one example of the many kinds of work engaged in to better manage or cope with prognosis—and, ultimately, to live with a chronic condition. This is a theme that has long been a central concern for those working in medical sociology and anthropology (Rier 2010, 130; Kleinman 1988, 4). However, Arthur Kleinman reminds us that it is ‘uncertain what successful coping means in any generic sense’ but suggests that it is ‘not something that can be achieved outright, once and for all. Patients and families, and, what is more, practitioners too, struggle to cope on a daily basis’ (1988, 144). This article, then, presents an example of one kind of continuous (in fact, lifelong) struggle to cope, in this case, with the prognoses of CHDs in outpatient encounters. Given the developments I have outlined, outpatient encounters and the prognostic calibrations taking place within them will most likely become a mainstay of living chronically in the 21st century and thus should become an increasingly central theme in medical anthropology.
Acknowledgements
I would like to express my sincere gratitude to all the children and young people with CHDs and their families, who allowed me a glimpse into their lives and to the healthcare staff who allowed me to complete my fieldwork in their hospitals. I would also like to thank Ayo Wahlberg, Signe Lindgård Andersen, Gunnar Gislason, Mia Jess, and colleagues in the Health and Life Conditions research group at the Department of Anthropology, University of Copenhagen, as well as the members of my PhD assessment committee (Susan Reynolds Whyte, Myra Bluebond-Langner, and Gitte Wind), for their valuable comments on early drafts of this article. I would like to thank the Children’s Heart Foundation, Denmark, and the Innovation Fund, Denmark, for funding the doctoral research on which this article builds. Further work on the article was supported by the European Research Council Starting Grant ‘The Vitality of Disease—Quality of Life in the Making’ (Grant No. 639275).
About the author
Marie Kofod Svensson is a medical anthropologist and postdoctoral researcher at the Danish Heart Foundation. Her doctoral research examined how Danish families live with congenital heart defects (CHD) for the Danish Heart Foundation (in collaboration with the Department of Anthropology, University of Copenhagen). Besides looking at how families navigate and adjust their understandings of prognoses, her doctoral work has also documented the chronic paradoxes that life with CHD can entail and work related to the (in)visibility of defect hearts. In her current research, she is exploring parental experiences of prenatal diagnosis of CHDs in Denmark.
Footnotes
-
All names are pseudonyms. Other details have also been changed or omitted to preserve anonymity (e.g., rather than describing specific CHD diagnoses, I have, together with a medical researcher on CHDs, categorised them as simple, moderate, or complex, building on Warnes et al. [2008, e154-e155] and descriptions of diagnoses, symptoms, and treatments provided by the families).↩︎
-
All doctors in this article are paediatric cardiologists. I have omitted their titles and genders, as well as the nurses’, to ensure anonymity.↩︎
-
I asked the families’ permission to observe check-ups and posted flyers about my project in the clinics. All interactions and interviews took place in Danish and have been translated by the author.↩︎
-
Twenty-two parents (six fathers, 16 mothers), eight children and young people with CHDs (five boys, three girls), seven siblings (all girls), and one grandparent (a grandmother). As, in most families, neither siblings nor grandparents regularly came along to check-ups, they are not the focus of this article. However, it could be interesting to explore how such exclusion affects siblings’ understanding of the CHD and its prognosis, as it is likely to make their involvement in the family dynamics related to the CHD even more complicated.↩︎
-
Overall, most fieldwork took place between the spring of 2016 and the spring of 2018 (clinical fieldwork in 2016). However, three pilot interviews took place in 2015, one follow-up interview in 2019, and two follow-up observations took place in clinics in 2017.↩︎
-
An examination to find out how well the heart is working through a catheter into a large blood vessel leading to the heart (American Heart Association 2015).↩︎
-
Other examinations, tests, and measurements are done too, such as objective physical examinations, blood tests, cardiac stress tests, auscultation, measurements of height and weight, and ECG and saturation readings. However, as echocardiography is often most in focus, I concentrate on this. The image of an echocardiography in process in an outpatient clinic was not taken during my fieldwork and does not portray any of the informants mentioned in this article.↩︎
-
Both studies are more than 10 years old and, given that estimating prognosis for individual patients with CHDs is challenging, I am aware that the prognostic estimates used as a comparison in these studies are quite uncertain. Nevertheless, the numbers seem so conspicuous that I believe they are relevant at least for reflection.↩︎
References
Alderson, Dorothea P. 1988. ‘Informed Consent: Problems of Parental Consent to Paediatric Cardiac Surgery’. PhD diss., Goldsmith’s College.
American Heart Association. 2015. ‘Cardiac Catheterization’. Web. July 31. https://www.heart.org/en/health-topics/heart-attack/diagnosing-a-heart-attack/cardiac-catheterization.
American Heart Association. 2018. ‘About Congenital Heart Defects’. Web. May 8. https://www.heart.org/en/health-topics/congenital-heart-defects/about-congenital-heart-defects.
Blaxter, Mildred. 2009. ‘The Case of the Vanishing Patient? Image and Experience’. Sociology of Health & Illness 31 (5): 762–78. https://doi.org/10.1111/j.1467-9566.2009.01178.x.
Bluebond-Langner, Myra. 1980. The Private Worlds of Dying Children. Princeton, NJ: Princeton University Press.
Bluebond-Langner, Myra. 1996. In the Shadows of Illness: Parents and Siblings of the Chronically Ill Child. Princeton, NJ: Princeton University Press.
Bouma, Berto J., and Barbara J. M. Mulder. 2017. ‘Changing Landscape of Congenital Heart Disease’. Circulation Research 120 (6): 908–22. https://doi.org/10.1161/CIRCRESAHA.116.309302.
Cambridge English Dictionary. n.d. (a) ‘Calibrate’. https://dictionary.cambridge.org/us/dictionary/english/calibrate.
Cambridge English Dictionary. n.d. (b) ‘Prognostication’. https://dictionary.cambridge.org/us/dictionary/english/prognostication.
Chessa, Massimo, Gabriella De Rosa, Manuela Pardeo, Diana G. Negura, Gianfranco Butera, Alessandro Giamberti, Edoardo Bossone, and Mario Carminati. 2005. ‘What Do Parents Know about the Malformations Afflicting the Hearts of Their Children?’ Cardiology in the Young 15 (2): 125–29. https://doi.org/10.1017/S1047951105000284.
Christakis, Nicholas A. 1999. Death Foretold: Prophecy and Prognosis in Medical Care. Chicago, IL: The University of Chicago Press.
Fonseca, Claudia, Soraya Fleischer, and Taniele Rui. 2016. ‘The Ubiquity of Chronic Illness’. Medical Anthropology 35 (6): 588–96. https://doi.org/10.1080/01459740.2016.1194411.
Gammeltoft, Tine M. 2013. ‘Potentiality and Human Temporality: Haunting Futures in Vietnamese Pregnancy Care’. Current Anthropology 54 (S7): S159–71. https://doi.org/10.1086/670389.
Guell, Cornelia. 2007. ‘Painful Childhood: Children Living with Juvenile Arthritis’. Qualitative Health Research 17 (7): 884–92. https://doi.org/10.1177/1049732307305883.
Heinsen, Laura L., Ayo Wahlberg, and Helle V. Petersen. Forthcoming. ‘Surveillance Life and the Shaping of “Genetically at Risk” Chronicities in Denmark’. Anthropology and Medicine.
Hjerteforeningen. n.d. ‘Medfødt Hjertefejl’. Hjertetal. https://hjerteforeningen.shinyapps.io/HjerteTal/?_inputs_&agCHD=%22age%22&bar=%22chd%22&oCHD=%224%22&var_chd=%22count_n_prevalence%22.
Jacobsen, Joes R., Keld Sørensen, Jørgen Videbæk, and Lars Søndergaard. 2010. ‘Arbejdsgruppen for Medfødte Hjertesygdomme’. https://www.cardio.dk/dcs-50-ars-jubilaeumsberetning-2014.
Jain, Sarah L. 2007. ‘Living in Prognosis: Toward an Elegiac Politics’. Representations 98 (1): 77–92. https://doi.org/10.1525/rep.2007.98.1.77.
Jenkins, Kathy J. 2017. ‘Congenital Heart Defects: Amazing Advances and Ongoing Challenges’. Journal of the American College of Cardiology 69 (22): 2733–34. https://doi.org/10.1016/j.jacc.2017.04.008.
Jenkins, Richard, Hanne Jessen, and Vibeke Steffen. 2005. Managing Uncertainty: Ethnographic Studies of Illness, Risk and the Struggle for Control. Copenhagen: Museum Tusculanum Press.
Kendall, Lynne, Patricia Sloper, Robert J. P. Lewin, and Jonathan M. Parsons. 2003 (a). ‘The Views of Parents Concerning the Planning of Services for Rehabilitation of Families of Children with Congenital Cardiac Disease’. Cardiology in the Young 13 (1): 20–27. https://doi.org/10.1017/S1047951103000052.
Kendall, Lynne, Patricia Sloper, Robert J. P. Lewin, and Jonathan M. Parsons. 2003 (b). ‘The Views of Young People with Congenital Cardiac Disease on Designing the Services for Their Treatment’. Cardiology in the Young 13 (1): 11–19. https://doi.org/10.1017/S1047951103000040.
Kleinman, Arthur. 1988. The Illness Narratives: Suffering, Healing, and the Human Condition. New York City, NY: Basic Books.
Knowles, Rachel Louise, Valerija Tadic, Ailbhe Hogan, Catherine Bull, Jugnoo Sangeeta Rahi, Carol Dezateux, and UK Collaborative Study of Congenital Heart Defects (UKCSCHD). 2016. ‘Self-Reported Health Experiences of Children Living with Congenital Heart Defects: Including Patient-Reported Outcomes in a National Cohort Study’. PLOS ONE 11 (8): e0159326. https://doi.org/10.1371/journal.pone.0159326.
Langstrup, Henriette. 2013. ‘Chronic Care Infrastructures and the Home’. Sociology of Health & Illness 35 (7): 1008–22. https://doi.org/10.1111/1467-9566.12013.
Larsen, Signe H., Morten Olsen, Kristian Emmertsen, and Vibeke E. Hjortdal. 2017. ‘Interventional Treatment of Patients with Congenital Heart Disease’. Journal of the American College of Cardiology 69 (22): 2725–32. https://doi.org/10.1016/j.jacc.2017.03.587.
Leder, Drew. 1990. The Absent Body. Chicago, IL: The University of Chicago Press.
Linde, Denise van der, Elisabeth E. M. Konings, Maarten A. Slager, Maarten Witsenburg, Willem A. Helbing, Johanna J. M. Takkenberg, and Jolien W. Roos-Hesselink. 2011. ‘Birth Prevalence of Congenital Heart Disease Worldwide’. Journal of the American College of Cardiology 58 (21): 2241–47. https://doi.org/10.1016/j.jacc.2011.08.025.
Long, Debbi, Cynthia Hunter, and Sjaak Van Der Geest. 2008. ‘When the Field is a Ward or a Clinic: Hospital Ethnography’. Anthropology & Medicine 15 (2): 71–78. https://doi.org/10.1080/13648470802121844.
Løvschal-Nielsen, Pia, Rikke S. Andersen, and Lotte Meinert. 2017. ‘Dealing with the Uncertainty of Childhood Cancer’. Tidsskrift for Forskning i Sygdom og Samfund (14) 27: 99–115. https://doi.org/10.7146/tfss.v14i27.103057.
Lüscher, Thomas F. 2017. ‘Congenital Heart Disease: Some Progress, but Still the Challenge of a Lifetime!’ European Heart Journal 38 (26): 2021–23. https://doi.org/10.1093/eurheartj/ehx372.
Manderson, Lenore, Elizabeth Cartwright, and Anita Hardon. 2016. ‘Changing Childhoods’. Manderson, Lenore, Elizabeth Cartwright, and Anita Hardon, eds. The Routledge Handbook of Medical Anthropology, 18–42. New York: Routledge.
Mattingly, Cheryl. 2010. The Paradox of Hope: Journeys through a Clinical Borderland. Berkeley, CA: University of California Press.
Maynard, Ronald J. 2006. ‘Controlling Death—Compromising Life: Chronic Disease, Prognostication, and the New Biotechnologies’. Medical Anthropology Quarterly 20 (2): 212–34. https://doi.org/10.1525/maq.2006.20.2.212.
McLaughlin, Janice, and Edmund Coleman-Fountain. 2014. ‘The Unfinished Body: The Medical and Social Reshaping of Disabled Young Bodies’. Social Science & Medicine 120: 76–84. https://doi.org/10.1016/j.socscimed.2014.09.012.
Obas, Katrina A., Jessica M. Leal, Michele Zegray, and Janet E. Rennick. 2016. ‘Parental Perceptions of Transition from Intensive Care Following a Child’s Cardiac Surgery’. Nursing in Critical Care 21 (3): e1–9. https://doi.org/10.1111/nicc.12202.
Olsen, Morten. 2010. ‘Prognosis for Danish Patients with Congenital Heart Defects: Mortality, Psychiatric Morbidity, and Educational Achievement’. PhD diss., Aarhus University.
Perrin, James M., L. Elizabeth Anderson, and Jeanne Van Cleave. 2014. ‘The Rise in Chronic Conditions among Infants, Children, and Youth Can Be Met with Continued Health System Innovations’. Health Affairs 33 (12): 2099–2105. https://doi.org/10.1377/hlthaff.2014.0832.
Rapp, Rayna. 2000. Testing Women, Testing the Fetus: The Social Impact of Amniocentesis in America. New York City, NY: Routledge.
Re, Jennifer, Suzanne Dean, and Samuel Menahem. 2013. ‘Infant Cardiac Surgery: Mothers Tell Their Story: A Therapeutic Experience’. World Journal for Pediatric and Congenital Heart Surgery 4 (3): 278–85. https://doi.org/10.1177/2150135113481480.
Reid, Graham J., Gary D. Webb, Mor Barzel, Brian W. McCrindle, M. Jane Irvine, and Samuel C. Siu. 2006. ‘Estimates of Life Expectancy by Adolescents and Young Adults with Congenital Heart Disease’. Journal of the American College of Cardiology 48 (2): 349–55. https://doi.org/10.1016/j.jacc.2006.03.041.
Reventlow, Susanne Dalsgaard, Lotte Hvas, and Kirsti Malterud. 2006. ‘Making the Invisible Body Visible. Bone Scans, Osteoporosis and Women’s Bodily Experiences’. Social Science & Medicine 62 (11): 2720–31. https://doi.org/10.1016/j.socscimed.2005.11.009.
Rier, David A. 2010. ‘The Patient’s Experience of Illness’. Bird, Chloe E., Peter Conrad, Allen M. Fremont, and Stefan Timmermans, eds. Handbook of Medical Sociology, Sixth Edition, 163–78. Nashville, TN: Vanderbilt University Press.
Rosenwein, Stine Vork, Anne Sidenius, Teresa Holmberg, Susan Ishøy Michelsen, and Tine Tjørnhøj-Thomsen. 2019. Ungdomslivet med en Hjertesygdom: En Litteraturgennemgang og Kvalitativ Undersøgelse af de Unges Hverdag og Mødet med Sundhedsvæsenet og Uddannelsessystemet. Copenhagen: Statens Institut for Folkesundhed. https://www.sdu.dk/da/sif/rapporter/2019/ungdomslivet_med_en_hjertesygdom.
Russ, Ann J., and Sharon R. Kaufman. 2006. ‘Family Perceptions of Prognosis, Silence, and the “Suddenness” of Death’. Culture, Medicine and Psychiatry 29 (1): 103–23. https://doi.org/10.1007/s11013-005-4625-6.
Røpcke, Diana M. 2016. Hjertet. Aarhus: Aarhus Universitetsforlag.
Shim, Janet K., Ann J. Russ, and Sharon R. Kaufman. 2007. ‘Clinical Life: Expectation and the Double Edge of Medical Promise’. Health: An Interdisciplinary Journal for the Social Study of Health, Illness and Medicine 11 (2): 245–64. https://doi.org/10.1177/1363459307074696.
Street, Alice. 2014. Biomedicine in an Unstable Place: Infrastructure and Personhood in a Papua New Guinean Hospital. Durham, NC: Duke University Press.
Svensson, Marie Kofod, Ayo Wahlberg, and Gunnar H. Gislason. 2020. ‘Chronic Paradoxes: A Systematic Review of Qualitative Family Perspectives on Living with Congenital Heart Defects’. Qualitative Health Research 30 (1): 119–132. https://doi.org/10.1177/1049732319869909.
Sundhedsstyrelsen. 2016. ‘Samling af Børnehjertekirurgi i Danmark’. Web. 28 January. https://www.sst.dk/da/Feeds/~/media/8FD598791ED04CB58939AF379D0AA3D9.ashx.
Taussig, Karen-Sue, Klaus Hoeyer, and Stefan Helmreich. 2013. ‘The Anthropology of Potentiality in Biomedicine: An Introduction to Supplement 7’. Current Anthropology 54 (S7): S3–14. https://doi.org/10.1086/671401.
Taylor, Janelle S. 2008. The Public Life of the Fetal Sonogram: Technology, Consumption, and the Politics of Reproduction. New Brunswick, NJ: Rutgers University Press.
Thomi, Mirjam, Jean-Pierre Pfammatter, and Elisabeth Spichiger. 2019. ‘Parental Emotional and Hands-on Work—Experiences of Parents with a Newborn Undergoing Congenital Heart Surgery: A Qualitative Study’. Journal for Specialists in Pediatric Nursing 24 (4): e12269. https://doi.org/10.1111/jspn.12269.
Timmermans, Stefan, and Tanya Stivers. 2018. ‘Clinical Forecasting: Towards a Sociology of Prognosis’. Social Science & Medicine 218: 13–20. https://doi.org/10.1016/j.socscimed.2018.09.031.
Vonder Muhll, Isabelle, Gordon Cumming, and Michael A. Gatzoulis. 2003. ‘Risky Business: Insuring Adults with Congenital Heart Disease’. European Heart Journal 24 (17): 1595–1600. https://doi.org/10.1016/S0195-668X(03)00346-4.
Warnes, Carole A., Roberta G. Williams, Thomas M. Bashore, John S. Child, Heidi M. Connolly, Joseph A. Dearani, Pedro del Nido, James W. Fasules, Thomas P. Graham Jr, Ziyad M. Hijazi, Sharon A. Hunt, Mary E. King, Michael J. Landzberg, Pamela D. Miner, Martha J. Radford, Edward P. Walsh, Gary D. Webb. 2008. ‘ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease’. Journal of the American College of Cardiology 52 (23): e143–263. https://doi.org/10.1016/j.jacc.2008.10.001.
Whyte, Susan Reynolds. 2002. ‘Subjectivity and Subjunctivity. Hoping for Health in Eastern Uganda’. Werbner, Richard, ed. Postcolonial Subjectivities in Africa, 171–90. London: Zed Books.
Wolf-Meyer, Matthew, and Celina Callahan-Kapoor. 2017. ‘Chronic Subjunctivity, or, How Physicians Use Diabetes and Insomnia to Manage Futures in the United States’. Medical Anthropology 36 (2): 83–95. https://doi.org/10.1080/01459740.2016.1180294.
Worthington, Nancy H. 2015. ‘Where There Is a Doctor: An Ethnography of Pediatric Heart Surgery Missions in Honduras’. PhD diss., Columbia University.
Ytting, Linnea, and Lone Friis Thing. 2013. ‘Faderskap og Familieliv under Hospitalisering af Hjertesyge Børn’. Nordic Journal for Masculinity Studies 8 (1): 77–92.
Zheleva, Bistra, and J. Brian Atwood. 2017. ‘The Invisible Child: Childhood Heart Disease in Global Health’. The Lancet 389 (10064): 16–18. https://doi.org/10.1016/S0140-6736(16)32185-7.