An expanding class of mega-philanthropic institutions, most often based in the Global North but increasingly based in the Global South, has emerged as a driving force in global health. Among them, the Carlos Slim Foundation—located in Mexico City and funded by Mexican-born telecommunications tycoon Carlos Slim Helú—has spearheaded an ongoing strategy to boost chronic disease prevention in Mexico, principally through the development and promotion of a series of cutting-edge diagnostic tests. In this research article I trace the Foundation’s efforts to develop these technologies and integrate them into Mexican health policy. With these technologies serving as powerful conduits of the Foundation’s epistemic power, I show that Carlos Slim’s philanthropic investments are reshaping goals in the public health field and fostering new understanding of chronic disease risk among health officials and experts in Mexico and beyond.
Detecting Diabetes Risk
Philanthrocapitalism, Diagnostic Innovation, and Epistemic Power in Mexico
—
Abstract
Introduction
Telecommunications magnate Carlos Slim Helú’s impact on Mexican life, from its most intimate to its most public aspects, is difficult to overstate. In 2010, a reporter for The Guardian observed that it is
virtually impossible for Mexicans to go about their lives without in some way contributing to his fortune … They are born in Slim’s hospitals, drive on his Tarmac, smoke his tobacco. They build their houses from his cement, eat in his restaurants, talk on his phones, and sleep in bed linen made in his factories (Harris 2010, para. 12).
Also acknowledging Slim’s commercial omnipresence, journalist Diego Enrique Osorno noted in 2015 that in Mexico:
Opinion on [Slim] is divided between the indulgence of intellectuals, politicians and artists, who see him as a nationalist benefactor, and fierce attacks from ordinary citizens, who have no other choice than to be his customers because he owns the vast majority of the products and services they consume (2015, 41).
Despite this divided opinion, most people in Mexico agree that, from the point of view of the sheer volume of wealth he has amassed over his lifetime, Carlos Slim is an entrepreneurial mastermind. He was already considered one of Mexico’s most elite entrepreneurs when he bought Mexico’s telephone services when they were privatised in 1990. That purchase catapulted him into a mobile phone and internet empire that today spans the Americas. With myriad other holdings across banking, construction, civil infrastructure, media, retail, pharmacies, medical laboratories, and private hospitals, he has for decades held the rank of richest person in Mexico. Between 2010 and 2013 he was also ranked the wealthiest person in the world. More recently, in 2020, he was ranked 15th globally with a fortune of over US$51.2 billion.
In the 1980s, Carlos Slim created two major philanthropic foundations. In 2007 he began to double down on these investments, pledging to increase his charities’ endowments from US$4 billion to US$8 billion over the following four years (Braine 2007). This would include a significant expansion of the Carlos Slim Foundation’s health sector, with an initial US$500 million committed to tackling Latin America’s most pressing public health concerns. With this investment, Slim joined the ranks of an elite cadre of tech giants-turned-philanthropists—most of whom hail from the Global North—who have become a driving force in the enterprise of global health, among other social welfare sectors (McGoey 2015; Fejerskov 2017; Mahajan 2018; Birn 2014; Al Dahdah 2019).
Central to the Carlos Slim Foundation’s public health agenda was Mexico’s growing epidemic of chronic disease. It also undertook initiatives aimed at maternal and child health and the eradication of dengue and malaria in southern Mexico and Central America, which it tackled in partnership with the Bill and Melinda Gates Foundation, the Inter-American Development Bank, and others. However, in contrast to peer institutions like the Gates Foundation, which typically funded external proposals for public health initiatives, the Carlos Slim Foundation assumed the role of a ‘think tank’, analysing health needs in the region and funding solutions of its own design.
Invoking a ‘social investment’ approach, the Foundation’s representatives also aimed to foster public health by supporting innovation in the private sector, funding critical research for new technological developments with an eye to their commercialisation. In a talk at the University of California Irvine in 2010, the head of the Foundation’s health sector and later its general director, Dr Roberto Tapia-Conyer, explained that with this approach the Foundation would ‘share risk’ with private investors (Tapia-Conyer 2011). Through such partnerships, Dr Tapia-Conyer continued, the objective of this ‘social business model’ was to make technological innovations for public health widely available in national contexts like Mexico, where the cost of their wider implementation in the public sector might otherwise be unaffordable.
Significantly, the private investors above include Carlos Slim himself, whose corporation has also invested in the commercial side of the Foundation’s endeavours. Carlos Slim thus ranks among those contemporary philanthropists increasingly referred to as ‘philanthrocapitalists’. As Linsey McGoey (2015) observed, this contemporary breed of philanthropists is notably ‘proud, triumphant even’ about the private economic gains to be made alongside their charitable donations, and among them it is ‘no longer necessary to “disguise” or minimize self-interest’ (ibid., 20). Accordingly, the Carlos Slim Foundation’s philanthropic initiatives have promoted digital and communications technology as a necessary infrastructure for public health advances, explicitly linking health gains to the kinds of technologies that have fuelled the meteoric increase in Slim’s personal fortune, as well as to emerging technologies in which his corporation has newly invested.
Specifically, the Foundation has pursued a plan to develop and integrate a series of new preventative diagnostic technologies into the Mexican public health system, to tackle chronic diseases such as heart disease and diabetes—Mexico’s leading causes of death. These technologies are designed to equip patients and healthcare providers with an understanding of individual chronic disease ‘risk profiles’ and to guide clinic-based efforts at prevention. Further, by pooling the risk data from screening activities carried out by public clinics nationwide into an interactive digital database, the Foundation aims to facilitate the national health system’s electronic surveillance and management of those deemed at ‘high risk’.
In this article, I trace the Carlos Slim Foundation’s efforts to develop these new diagnostic technologies as ‘social investment’ and to integrate them into Mexican health policy. Below, I consider what the technologies may mean for Carlos Slim’s own bottom line and for the fortunes of other commercial actors capitalising on medicalised approaches to chronic disease prevention. More critically, I point to the influence that Carlos Slim is bringing to bear, through the development and promotion of these technologies, on the knowledge and information available in the arenas of medicine and public health in Mexico. In doing so, I highlight the epistemic power that Carlos Slim wields through his philanthropic investments and the medical diagnostic technologies they have supported. Differentiating epistemic power from compulsory power in the domain of public health, Jeremy Shiffman (2014) has written that epistemic power is productive; that it is the power at work in the creation of concepts used for thinking about health priority-setting. I show that beyond funding a set of initiatives and programmes, Carlos Slim’s philanthropic investments are re-shaping the goals of the public health field and fostering new understanding of chronic disease risk among health officials and experts in Mexico and beyond.
In the following section, I briefly situate the Carlos Slim Foundation as an exemplar among contemporary philanthrocapitalists in the arena of global health. Next, I explain the development of his diagnostic technologies and track their initial integration into Mexico’s health system. I conclude with an analysis of their epistemic implications. My analysis draws on 20 months of multi-sited ethnographic fieldwork carried out primarily in Mexico City between 2016 and 2019, entailing extended observation in public and private primary care clinics, attendance at key national and international scientific conferences, and 65 in-depth interviews with health officials, healthcare providers, scientific researchers, health activists, and representatives of the Carlos Slim Foundation.
Philanthrocapitalism and technological innovation for health
Carlos Slim’s approach to health philanthropy parallels that of a growing class of mega-philanthropists—perhaps most famously among them, Bill Gates—who have similarly made their fortunes in the tech sector and are today transforming the field of global health. Proponents of these elite donors, popularly termed ‘philanthrocapitalists’, champion the ideas that (1) philanthropy should be practised with the efficiency of for-profit businesses and therefore is appropriately overseen by successful entrepreneurs; and, that (2) capitalism intrinsically drives innovation that is beneficial to society (Bishop and Green 2008).
Meanwhile, among social scientists the influence of donors lauded as philanthrocapitalist exemplars has increasingly drawn criticism (see especially, McGoey 2015; Fejerskov 2017; Mahajan 2018; Birn 2014; Al Dahdah 2019). With regard to the extreme accumulation of wealth that underpins big philanthropy, such critics highlight three central concerns: first, the funds wielded by philanthropists are diverted from public coffers (and are typically no longer subject to taxation or to forms of democratic debate about the ends to which they are put and how they will be distributed); second, as such, these funds are exempt from the accountability and transparency to which public budgets are typically subject; and third, these funds are often amassed via corporate practices that simultaneously work against the social justice goals that philanthropists expound (Birn 2014; McGoey 2015). For example, in Carlos Slim’s case, the telecommunications tycoon has been accused of exerting a dangerously monopolistic hold over Mexico’s telecommunication infrastructure and, thereby, of stifling the nation’s overall development and global competitiveness (Thompson 2006).
Scholars also increasingly point to the epistemic power that philanthrocapitalists exert. It is their values, their chosen research questions, strategies, and metrics that now overwhelmingly direct global health action (McGoey and Thiel 2018; Mahajan 2019; Reubi 2018). Moreover, as Adam Moe Fejerskov (2017) has observed, their approaches to global health are consistently guided ‘by logics of the individual, the market, and of societal progress through technological innovation’ (948). Indeed, the influence of contemporary elite philanthropists has entailed a kind of second coming of technology in the fields of global health and development (ibid., 2017), with particular emphasis on digital communication technology, advanced informatics for ‘big data’ analysis, and experimental applications of vanguard science.
Importantly, philanthrocapitalists and the technological innovations they promote are often received in expert circles and across publics as having unquestioned technical and moral authority. This is due to both the tremendous success of their for-profit, tech-sector endeavours and the benevolence and self-sacrifice that is widely linked to philanthropic giving in the public imagination (McGoey and Thiel 2018)—this, even as their on-the-ground experimentation in vulnerable settings is often marked by the acceptance of ‘constructive failures’, reflecting many of these philanthropists’ ties to Silicon Valley and their endorsement of the ‘fail better’ mantra famously pervasive there. Certainly, among Mexicans, the Carlos Slim Foundation is widely perceived to be far more technically and ethically competent than the state itself, even as its investments in health have fallen short of its stated goals. As one physician reminded me, emphasising the scarcity of state resources for chronic disease prevention and medical services more broadly: ‘At least Slim is doing something’.
Thus, all this is not to say that the contemporary surge in global health philanthrocapitalism among the mega-rich is necessarily without worth. Extraordinary benefits may come of their philanthropic gifts. Rather, through my observations and interviews with the interlocutors described above, my aim here is to make clear that as global health challenges are increasingly placed under the stewardship of private philanthropic institutions like the Carlos Slim Foundation, we are faced with an important reckoning regarding their influence—the privileging of their values, interests, and tools—over how we collectively recognise health and how we conceive potential avenues for its attainment.
Further, in Mexico, representatives of the Carlos Slim Foundation have promoted the organisation’s initiatives and philosophy through a nationalist lens. By doing so, they have largely circumvented criticisms levied at peer institutions such as the Bill and Melinda Gates Foundation, which are frequently painted as external, imperialist threats. Cultivating the Foundation’s profile as a local (and extremely capable) health sector partner, its representatives have established largely unchallenged close relationships with the Mexican government over the last decade. Indeed, a veritable revolving door has existed between the Foundation and the upper echelons of Mexican government, as high-level health officials have assumed key leadership positions in the Foundation upon leaving office. Thus, unsurprisingly, in an interview conducted in 2018, the then secretary of health, Dr José Narro Robles, described his secretariat’s relationship with the philanthropic institution, as follows: ‘We have an extraordinary alliance with the Foundation. There is a symbiosis, a sum of capabilities, of possibilities’. In this context, the institution’s ideologies, interests, and epistemic influence uniquely overlap with the evolving contours of the Mexican state’s social contract with its citizens in the arena of public welfare.
Detecting diabetes risk in Mexico
The Carlos Slim Foundation’s chronic disease prevention efforts took shape as Mexico’s epidemic of chronic diseases burgeoned over recent decades, culminating in 2016 in the declaration of diabetes and obesity as a national health emergency. These were the first ever non-infectious conditions to achieve emergency status in Mexico. By the early 2000s it was evident that rates of diabetes in the country had begun to climb sharply (Barquera et al. 2003). By 2005 the World Health Organization had recognised that the developing world was and would continue bearing the highest impact of chronic disease globally, with four out of every five chronic disease deaths taking place in low- or middle-income countries. This was the epidemiological outlook for Mexico that representatives of the Foundation aimed to challenge, as I describe below.
The MIDO programme: Screening for risk and disease
In 2010, the Foundation launched the ‘Integrated Measurement for Early Detection’ programme, known by its Spanish acronym MIDO (also literally meaning ‘I measure’). The MIDO programme entailed the installation in primary healthcare clinics (or centros de salud) of an ‘all-in-one’ diagnostic station for chronic disease screening and counselling, as well as an electronic information system that enables long-term patient monitoring. A mobile version of MIDO allows screening to take place away from clinics. Alongside these tools there is an online platform providing relevant continuing education for healthcare providers, as well as a system-wide database that the Foundation curates to support health system planning (Tapia-Conyer et al. 2017).
MIDO was intended to target apparently healthy patients (including family members accompanying sick patients to health clinics), rapidly screening them for chronic diseases, pre-diseases (such as pre-diabetes and pre-hypertension), and traditional risk factors (including elevated BMI, abnormal blood sugar levels, high cholesterol, and family history of disease). In this way, according to Foundation representatives, the MIDO system was designed to resist the dichotomisation of screened individuals into ‘healthy’ or ‘sick’, and instead to promote an understanding of pre-disease stages so that healthcare providers and patients could treat ‘risk’ rather than illness only (Tapia-Conyer, Gallardo-Rincón, and Saucedo-Martinez 2013). Treatments to reduce or control individuals’ risk might involve recommendations for changes to their lifestyles, including diet and exercise. In the case of pre-diabetes, this advice might also be accompanied by a prescription for metformin—a first-line diabetes drug used to treat the initial stages of diabetes.
The Foundation’s representatives viewed the promotion of pre-diseases such as pre-diabetes as a way to jump-start a shift across Mexican society toward taking prevention seriously. ‘It’s shock therapy,’ one representative told me in an interview. ‘You need to give the patient a nudge so that he wakes up.’ In other words, the representative explained, the intention is to be able to tell individuals who are at risk: ‘Don’t think you’re healthy; you’re on the threshold of being sick’.
By 2017, the Foundation had rolled out the MIDO programme in 27 of Mexico’s 32 states. It had done so by building partnerships with state and local governments and supporting staff in the public clinics to implement the model. As the Foundation’s representatives explained to me, once these governments had incorporated the model into local health policy and practice, the Foundation’s goal was to leave the MIDO technology entirely in local hands. This proved not to be possible, however, nationwide. ‘What happens in the case of MIDO, is that it requires serious political commitment. You can imagine what the five states that we haven’t reached are—Chiapas, Oaxaca, Michoacán, Chihuahua, and Guerrero,’ the same representative said. The states he mentioned include Mexico’s poorest, marked by the most troubling health indicators in the country:
In Oaxaca, Guerrero, Chiapas, it’s very complicated. We have tried with maternal health, we’ve tried with vaccines, and everything systematically fails in those states. So, the problem is not MIDO; the problem is the state itself, which is not in a condition to adopt new projects and work to sustain them.
As a private rather than a government entity, the Foundation could decide to exclude these states—where quantitative evidence of the programme’s success would no doubt be elusive—at its own discretion. That said, it is clear that the technology-driven, metrics-oriented approach to chronic disease prevention it favoured was a mismatch for these areas of extreme poor health. According to this Foundation representative, the broader social challenges of acute poverty and unstable governance besetting these regions were beyond the scope of the Foundation’s expertise.
By 2018, in the states where the Foundation had implemented MIDO, the programme had screened more than a million individuals (Broadband Comission 2018). As the institution’s leaders reported, 40% of those screened would not have been so under previous policies, 13.4% were found to have pre-diabetes, and 5.8% had undiagnosed diabetes (Tapia-Conyer et al. 2017). Highlighting these results at the 2018 General Assembly of the United Nations, the Broadband Commission—a joint initiative of the United Nations Education, Scientific and Cultural Organization (UNESCO) and the International Telecommunication Union that promotes Internet access to help achieve the UN’s Millennium Development Goals—hailed the MIDO technology as an international reference point for the integration of digital solutions into health policies (Broadband Comission 2018).
The SIMGA initiative: Understanding the genetics of diabetes
In 2010, the same year in which MIDO was launched, the Carlos Slim Foundation announced a second strategy for confronting Mexico’s diabetes epidemic. Not satisfied with improving accessibility to screening for traditional risk factors through the MIDO programme, it would now turn to cutting-edge science to redefine the risk factors for which individuals would be screened. To this end, the Foundation donated US$135 million to establish the Slim Initiative for Genomic Medicine (or SIGMA). The aim of SIGMA was to accelerate research on the genetics of type 2 diabetes in Latin America, leveraging, according to Foundation’s representatives, the region’s ‘unique population genetics’ (Broad Institute n.d.; see also Vasquez and García Deister 2019). El Economista, a Mexican business and economics newspaper, reported this as the largest philanthropic investment in scientific research ever made in Mexico or Latin America (Toche and Lino 2014).
Pointing to Carlos Slim’s personal influence over the strategies pursued by the Foundation, General Director Dr Tapia-Conyer explained to me that its focus on cutting-edge genomic science grew out of Slim’s own interest in this emerging field:
Carlos Slim and his son Marco Antonio Slim are on top of what is new. They don’t just live in the day to day … [Carlos Slim] is truly visionary, I mean he anticipates scenarios that you don’t see yet, but he already does. And he started to ask us: ‘What is going on with chronic disease, with diabetes, genetics? Who is the best for that?’
The answer to his question was Dr Eric Lander, the then president and executive director of the Eli and Edyth L. Broad Institute (hereafter referred to as ‘the Broad’) at Harvard and MIT, a research centre at the forefront of genomic medicine globally.[1] Announcing the partnership that the Carlos Slim Foundation would go on to form with the Broad to conduct path-breaking work on the genomics of diabetes among Latin Americans, Dr Lander described the venture as a visionary engagement with public health, ‘First, in recognizing that progress in public health must be built on a foundation of scientific understanding of the genetic basis of disease. Second, in recognizing that deepening the scientific ties between the US and Mexico can have great benefits for both countries’ (Broad Institute 2010, 1).
The SIGMA initiative’s strategy was to link key researchers and institutions in Mexico to the Broad, which would serve as the central node of the SIGMA venture and accelerate the project’s pace of discovery. ‘We didn’t want to invest in basic research to create basic knowledge,’ Dr Tapia-Conyer explained. ‘We wanted to be able to convert that knowledge immediately into policy action.’ Indeed, urgency around translating the genetics of diabetes into technologies that would have clinical impact was at the heart of the venture, and the Foundation wanted progress on an entrepreneurial rather than an academic timescale. As one SIGMA scientist explained, Slim’s gift had come with a timeline for bench-to-bedside translation, i.e., the fast production of marketable technology. ‘He’s an entrepreneur,’ the scientist told me. ‘[Slim] said okay, I’m going to put in this amount of money, but after three years I’m going to get something out of it with potential commercial value.’
This goal of a fast return would also require infrastructure on the commercial side. Therefore, just as SIGMA was reporting its first findings the Foundation founded a biotechnology start-up firm, Patia Biopharma (hereafter referred to as ‘Patia’). Built primarily with funding from the for-profit arm of Carlos Slim’s conglomerate, the start-up’s offices were set up directly across the street from, and within view of, the Carlos Slim Foundation’s conference room in Mexico City. As Dr Tapia-Conyer explained to me, this arrangement was an experiment:
It’s an exercise, an initiative, something very innovative. To create a firm where investment, private investment, literally stockholders, are linked with social investment, literally a foundation. What you get is a business with a commercial but also a social vision at the same time, where its earnings are just sufficient, just above cost, for its own sustainability. The philosophy stems from another story from within the group, a construction firm that Slim designed to undertake large-scale projects in Latin America with profit margins just above cost. That firm has been very successful, so successful that this small fraction above cost has meant profit.
Patia and the Carlos Slim Foundation entered into a partnership, guided by the Foundation’s self-described ‘social business model’ approach, in which the two entities and their missions were deeply intertwined.[2] By 2014, Patia’s website contained a description of the start-up’s focus on the genomics of diabetes and cancer and its ‘commitment to close the gap between research and clinical implementation through the creation of preventive, diagnostic, and therapeutic tools within reach of the entire Mexican population, thus transforming health through “preventive, personalized, and social” genomic medicine’ (Patia Biopharma 2014). As a first step, the company had already become Mexico’s exclusive distributor of Myriad Genetics’s BRCAnalysis test, which detects BRCA1 and BRCA2 gene mutations linked to hereditary breast and ovarian cancer risk (Cruz Martínez 2013).
Quantose IR: Detecting the risk of being at risk
Patia’s next move was to enter the field of metabolomics—the study of small-molecule substances formed during metabolic processes or necessary for metabolism to take place (Metabolon 2017a). Originally developed by US biotech firm Metabolon Inc., the blood test Quantose IR measures the level of certain metabolites in the blood that together constitute a novel biomarker for insulin resistance (Cobb et al. 2013)—a condition that indicates that an individual’s cells are not responding normally to insulin. In 2014, the test was exclusively licensed to the Carlos Slim Foundation, Patia, and one Mexican laboratory, for its commercialisation in Mexico (Business Wire 2014).
When insulin resistance occurs, glucose cannot enter cells easily and instead begins to accumulate in the bloodstream. Rather than detecting the build-up of glucose in the blood, however, the Quantose IR technology uses mass spectrometry to measure levels of insulin and three other non-glycaemic (i.e., non-glucose-related) metabolites. These substances signal changes that ‘occur years before changes in haemoglobin A1C and fasting plasma glucose are visible’ (Metabolon 2017b). To be more specific, Quantose IR, as the company’s website explains, detects changes in insulin resistance that begin more than ten years prior to the glycaemic changes that a standard haemoglobin A1C test detects, when 70 to 80% of beta cell (or β-cell, the body’s key manufacturers of insulin) function in the pancreas has typically already been lost. In other words, Quantose IR’s innovation is that it can detect indicators of diabetes risk that appear long before traditional markers of risk do. Indeed, Patia’s website describes the test as the first technology available ‘permitting the identification with precision of individuals at risk of developing pre-diabetes and diabetes’ (Patia 2015). This suggests that Quantose IR can effectively diagnose the risk of a risk—i.e., it can identify a state prior to the risk state of pre-diabetes, when silently, imperceptibly, pathological changes are occurring in the body as it begins to advance toward ‘pre-disease’.
In the private clinics in Mexico City where I conducted my observations, the test is used not only for the early diagnosis of insulin resistance but also to assess a patient’s progress over time in lowering their level of diabetes risk, usually while undergoing pharmacological treatment. In these clinics, patients receive their Quantose IR result in the form of a print-out containing a horizontal bar—half green (on the left) and half red (on the right)—that extends across the page. The green zone corresponds to a score of 1 to 63, indicating ‘insulin sensitivity’, while the red zone corresponds to a score of 63 to 120, denoting ‘insulin resistance’ (see Figure 1).
Author’s Quantose IR result.
By design, Quantose IR allows for further improvement even after ‘insulin sensitivity’ has been achieved, i.e., a move deeper into the green zone away from the red zone. In this respect, even my own score of 10 (Fig. 1) suggests that although I am in good shape I could still do better. That said, a state of ‘no risk’ remains elusive. One year into my fieldwork observing primary care visits in clinics across Mexico City, one of the physicians who used Quantose IR in his private practice wrote to me, via WhatsApp: ‘See there, you can do it if you really try’. His message was accompanied by a photo of a Quantose RI result with a score of 1—an achievement neither he nor I had ever seen before. His patient, he explained, was a competitive gymnast.
DIABETESprevent: Testing for genetic predisposition
Following the introduction of Quantose IR, by 2014 the Foundation’s investments in genomic medicine through the SIGMA initiative had led to the discovery of two genetic variants linked to heightened risk for type 2 diabetes among Mexicans and Latin Americans (Vasquez and García Deister 2019). For many of the scientists involved, the discovery of this association represented a preliminary advance in knowledge in this area and a starting point for a programme of research to determine the variants’ functions. For example, in a press release, Dr Teresa Tusié Luna of the Mexican Institute of Health Sciences and Nutrition in Mexico was quoted as saying: ‘While the finding is associated with high levels of risk for the disease, we still do not know how the mutation behaves and if there is a specific therapeutic method that can be safely applied in the clinic for individuals that carry the mutation. There is much more we need to learn’ (Ruiz Jaimes 2014).
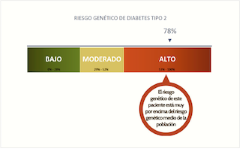
Nonetheless, on the basis of these findings and with the political backing of the Carlos Slim Foundation, Patia proceeded to develop and market a test that would allow Mexicans to determine their genetic predisposition to type 2 diabetes. The test, called DIABETESprevent, entails performing a cheek swab to collect a DNA sample. The sample is then tested for the presence of 16 different genetic variants, including the two discovered by SIGMA’s scientists. The test applies an algorithm based on the presence (or absence) of these variants to produce a ‘type 2 diabetes genetic risk score’. Patients receive their score either from their doctor or, if they have taken the test at home, directly via a smart-phone app. The app also doubles as a health management portal, through which users are encouraged to input and track data about their health, diet, and exercise to help them manage their risk.
Patia initially made DIABETESprevent available for purchase both through physicians and online as a direct-to-consumer product. At that point, it was apparently valued at 1,400 pesos (US$75) on the international market. Representatives of the Carlos Slim Foundation, however, announced that the institution was subsidising the sale of the test in Mexico and at its launch duly set a price of 999 pesos (about US$50 at the time). In the lead up to the launch, Patia’s homepage was redesigned to feature the tagline ‘You Can Prevent Type 2 Diabetes—Discover your and your family’s genetic predisposition to type 2 diabetes with a simple test’. Below this, an image of a smiling young family appeared—a fit, fair-skinned couple were pictured with a blond-haired, blue-eyed child (a racialised marketing approach exceedingly common in Mexico)—suggesting both that testing for genetic predisposition to diabetes is a family affair and that the test’s target market is the healthy and young. The company encouraged such cascade testing by offering a 20% discount on ‘family packs’.
While I was observing one physician in his private clinic on the outskirts of Mexico City, a 33-year-old man, Arturo,[3] arrived with his wife and a set of lab results. The results showed a haemoglobin A1C of 11.6% (a score of 6.5% is generally accepted as the cut-off point above which diabetes is diagnosed). ‘You definitively have very aggressive diabetes,’ the physician said, reviewing the lab results. ‘It’s serious and that’s why you’ve been losing weight. This needs serious attention.’ As the physician explained that he would need to prescribe insulin, Arturo, visibly upset by the news, sank into the couch flanking one wall of the examination room. He then asked two questions: ‘Why don’t I feel bad?’ and ‘What about my children—what can I do to be sure they don’t get it?’ The physician explained that the body can become accustomed to high blood sugar levels and that was why Arturo felt more or less normal even though he was seriously ill. In response to Arturo’s question about his children, the physician replied: “What is the best prevention? A balanced diet and exercise.’ An additional option, he continued, would be to find out early on whether the children were at high risk. He then went on to mention the new DIABETESprevent test and explained to Arturo how it worked.
It went unsaid, however, that if Arturo’s children received a high risk score on the genetic test, the doctor’s subsequent advice would remain virtually the same: to manage their diet and exercise and to consider periodically monitoring their blood sugar levels and other indicators of risk. The test’s risk score would indeed provide a tangible metric around which Arturo and his children could plan; never mind, however, that the score would still only represent a series of complex probabilities, probably more accurately captured and calculated through a simple (and cost-free) family health history assessment (Fitipaldi et al. 2018).
MIDOPlus: From technology to policy
Representatives of the Carlos Slim Foundation worked to win recognition for its technologies on the world stage at scientific conferences (framing these new risk diagnostics as the Foundation’s intellectual contribution), and from policymakers. Meanwhile, in Mexico the Foundation launched one of its most substantial deployments of both Quantose IR and DIABETESprevent to date, drawing on its deep-rooted connections with Mexico’s Secretariat of Health, particularly in the state of Puebla. It entailed a campaign to test over two thousand patients in primary health centres, in which the nation’s most vulnerable receive care.
Launched in 2016 and funded by the Foundation, the campaign made use of the Foundation’s pre-existing MIDO programme—extended in Puebla under the name MIDOPlus, and with both Quantose IR and the genetic test DIABETESprevent now incorporated into the screening model. While the Foundation subsidised the cost of this pilot initiative, this ‘philanthropic’ donation also represented an investment in future commercial returns for Patia. After all, data from the initiative would grow the company’s database substantially, helping to refine its proprietary algorithms. It would also contribute to the evidence base on which to demonstrate the utility of these diagnostic tests to the Secretariat of Health and other health institutions, to justify a scaling up of their deployment across and beyond Mexico (Betancourt 2018).
In a talk to public health students in early 2018, the Carlos Slim Foundation’s Director of Global Solutions, Dr Miguel Betancourt, presented the preliminary results of the MIDOPlus pilot campaign. Of the patients in the sample found not to be diabetic or pre-diabetic, he reported, 64% nonetheless exhibited insulin resistance, according to the highly sensitive Quantose IR test. Furthermore, he went on, among those whose haemoglobin A1C, body mass index, waist circumference, and blood pressure were all normal, 38% exhibited insulin resistance according to their Quantose IR score. In other words, their bodies were showing signs of the earliest detectable move toward developing diabetes—signs that would have been missed by traditional tests. Moreover, he told his audience, 17.7% of the more than two thousand patients studied had a ‘high’ genetic risk score according to the DIABETESprevent test, 41.6% a ‘medium’ risk score.
The idea, Dr Betancourt explained, was to convert this data into yet another algorithm—i.e., to combine the results of these tests with information on blood glucose, body mass index, waist circumference, and blood pressure—in order to create a personalised risk profile schema. This schema would stratify patients according to their risk, making it possible to tailor preventative measures to each of them. By summer 2018, the Foundation had prepared guidelines for physicians on to how to deal with 21 different risk profiles. As a result, patients would no longer simply be considered just sick or healthy, nor either sick, at risk, or healthy; instead, this series of 21 different profiles would define their location on a dynamic continuum of risk.
According to Foundation staff, the combination of Quantose IR and DIABETESprevent in the context of a tool like MIDOPlus, along with the tool’s potential application on a national level, represents a new prevention ‘paradigm’ not just for medicine but for public health more broadly. In his talk to public health students, Dr Betancourt explained that a new era is at hand, an era of ‘personalized public health’ (Betancourt 2018). Recognising the contradiction the term seemed to imply, he elaborated:
What I am going to tell you goes totally against what our public health professors have taught us since our first engagement with this discipline: that public health is about population dynamics. Here we’re going to the other extreme, to the most intimate, most individual aspect of each human being. You’re all going to raise your hands and say, ‘No, public health is by definition a population-level approach’. But no, genomics, and a few of the other fields we’ll see at work today, are enabling us to individualize risk, create profiles, and better characterize our population.
He went on to add: ‘This is public health. This is the National Centre for Prevention and Control of Disease handing out Metformin across the country. But we know who we need to give it to and in what moment, in accordance with their risk type’ (Betancourt 2018).
Ultimately MIDO, Quantose IR, DIABETESprevent, and MIDOPlus are the kind of technological innovations that prove possible a re-articulation of what public health can and should be (Tapia-Conyer 2014). This new paradigm of personalised prevention in public health entails population stratification through routine diagnostic testing, with clinical resources directed toward ‘active prevention’ (i.e., lifestyle intervention, and potentially, pharmaceutical treatment) among those predicted to be most at risk. The new paradigm would, of course, also support the expansion of markets for diagnostic technologies capable of detecting risk, and for individualised therapeutic products to deal with risk where it is found, as well as for related laboratory infrastructure.
Taken together, these diagnostic technologies and the vision for personalised prevention that they enable point to a new chapter in the move towards risk profiling in clinical settings—a trend that scholars have previously primarily tracked in the US and Western Europe. With the rise of surveillance medicine, the experience of risk and of disease have increasingly converged in these settings, as have the practices of treatment and prevention (Aronowitz 2009, 2015). Analysts have long pointed to the role of the pharmaceutical industry in driving this convergence (Dumit 2012; Greene 2007; Fosket 2010; Rosenberg 2009), as well as to the rise of genetic and genomic technologies in accelerating biomedical risk prediction (Rose 2007; Clarke et al. 2010, 2003; Timmermans and Buchbinder 2010). The case of the Carlos Slim Foundation brings to the fore the emerging role of elite, tech-minded philanthropists in the global diffusion of clinic-driven prevention.
Diagnostic technologies and epistemic power
As the analysis above indicates, the Carlos Slim Foundation has exerted significant power over the production of health knowledge and information in Mexico. It has achieved this by doing the following: (1) directly funding novel research on the aspects of chronic disease that it considers most useful (i.e., genetic and metabolomic biomarkers of risk); (2) broadening the accessibility of diagnostic technologies in the clinical setting that generate new knowledge and data about individuals’ risk; and (3) aggregating and curating this new data through digitalised platforms to guide ‘evidence-based’ policymaking and future health system reform, through the lens of the metrics the Foundation has made possible. Importantly, diagnostic technologies are at the core of this ecosystem of epistemic power—they are a crucial mechanism enabling the Foundation to exercise influence. Through them, the Foundation is able to cultivate and disseminate particular imaginaries (i.e., conceptual infrastructures) both for what constitutes actionable risk and for what public health action should aim to achieve in this era of epidemic chronic disease.
The implications of the Carlos Slim Foundation’s epistemic power, as it has been expressed through the MIDO Programme, Quantose IR, DIABETESprevent, and MIDOPlus, begin with the quantitative differentiation of risk and therefore the triaging of public health action across Mexico. While at first glance this approach appears more efficient, much more is at stake: these technologies and the risk stratification they make possible encourage ‘targeted’ action aimed at those most ‘at risk’ and carried out through the medical system rather than through structural interventions that could resolve population-level risk through comprehensive social action such as food policy reform. A project that reduces risk among those singled out as ‘on the edge of disease’ is very different from building a society that cultivates health.
Further, these technologies convey a powerful endorsement of both medical and individual agency for preventing chronic disease. Their sale and circulation rely on the conviction that the risk scores they produce will motivate individuals to adopt healthier behaviours (or adhere to a preventative drug regime), underplaying the structural constraints that continue to influence and limit behaviour choices (Parthasarathy 2014; Nelson and Robinson 2014; Lee 2017). In fact, the Foundation’s investments in these technologies have effectively worsened disparities in terms of the evidence that is available to tackle structural drivers of chronic disease—a clear example what David Hess has called the disproportionate influence of social elites over ‘the political opportunity structure of research funding’ (Hess 2015, 142). The authority of both Carlos Slim’s persona and the political connections of the Foundation’s leadership have effectively directed the Mexican medical and scientific communities’ attention away from structural conditions and toward genetic drivers and behavioural (if not pharmaceutical) solutions.
More broadly, under the vision of the Carlos Slim Foundation, public health action has increasingly centred on the state’s ability to provide routine laboratory testing and follow-up treatment (often pharmaceutical) to those deemed at ‘high risk’. Here, the social contract between the state and its citizens centres on facilitating the latter’s access to and consumption of an array of products (i.e., diagnostics and pharmaceuticals), as opposed to protecting them from the actual sources of harm rampant across Mexico’s obesogenic environment. Ultimately, under this model, chronic disease prevention in Mexico is emerging as a highly sustainable commercial industry—a market underpinned by perpetual demand for long-term, technology-driven medical surveillance and preventive drugs. With the distal drivers of chronic disease virtually untouched, Slim himself will profit, as will many other commercial investors, given the continuing stream of many thousands ‘at risk’ and in need of monitoring and ‘treatment’ for chronic disease prevention.
Conclusion
Echoing the core tenets of philanthrocapitalism, Carlos Slim told journalist Enrique Diego Osorno that the generation of wealth, if done properly, ‘generates employment, generates more wealth, generates services, and important assets for society’ (Osorno 2015, 39). Further enriching himself, but most importantly fomenting new markets, Carlos Slim believes, will lead to a better quality of life for many. Given this ethos, developing innovative diagnostic tests for chronic disease prevention makes sense. They are products, conceived under Carlos Slim’s discerning watch, that imply jobs, income, and spending; they may very well also lead to better health, by encouraging prevention efforts targeted at lowering the biological indicators of chronic disease risk. Conversely, the role of the processed food and sugar-sweetened beverage industries in driving Mexico’s metabolic crisis, for example, remains peripheral; and a hard look at what wider access to liveable wages might mean for creating healthier households continues to be averted. Observing Slim’s 78th birthday, a columnist for La Jornada newspaper wrote:
What goals could Slim have at 78? As is typical for magnates, it’s possible that he no longer loses sleep over making his next million. Probably, he worries about the mark he will leave on this country that has been so generous with him.
The columnist goes on to suggest a hypothetical societal legacy:
What would happen if Slim did an experiment and raised salaries to at least five times the minimum wage? ... Maybe other business owners would follow his example (Galván Ochoa 2018, 6).
It is certainly worth asking what impact such an alternative approach to ‘social investment’ might have on health across the nation. As public social welfare programming in Mexico increasingly converges with Carlos Slim’s vision for health and development, it is critical to challenge the re-conceptualisation of public health as a burgeoning commercial industry and to consider instead public health action to protect citizens’ bodies from commercial markets and the ill-effects of increasing wealth inequality.
Acknowledgements
I thank Alice Street for her intellectual leadership in the production of the MAT’s special issue on medical testing, diagnosis, and value, as well as for her generous guidance on this article. Thanks also to Cristina Moreno Lozano for her work in support of this special issue. This work was made possible through the mentorship of Alondra Nelson and Richard Parker, as well as by the critical insights of Amaya Perez-Brumer and the community created by the members of the Nutrire CoLab. An early version of this paper was presented at the 2018 AAA Annual Meeting in San Jose, CA, and was awarded the 2020 Rudolf Virchow Award (graduate student category) by the Critical Anthropology for Global Health Caucus of the Society for Medical Anthropology. Related fieldwork and analysis was supported by the National Science Foundation, Science, Technology and Society Division (Grant: SES - 1656224) and an ACLS/Mellon Dissertation Fellowship. In memory of Helen Jenkins Smetts.
About the author
Emily Vasquez is a Bridge to the Faculty Postdoctoral Fellow in the Department of Sociology at the University of Illinois Chicago. An ethnographer of science, medicine, and public health, her research examines how social inequalities are entangled with and reinforced by the production of expert knowledge in these fields.
Footnotes
-
Today, Dr Lander serves as top scientific advisor to US President Joe Biden, as director of the US Office of Science and Technology Policy.↩︎
-
A quick corporate genealogy supports this point: Patia Biopharma is primarily held by a venture-capital fund owned by Grupo Financiero Inbursa, which is in turn a subsidiary of Grupo Carso, the umbrella conglomerate synonymous with Carlos Slim’s corporate empire. See https://www.inbursa.com/storage/Comunicado-Banco-Inbursa-2T17.pdf.↩︎
-
A pseudonym.↩︎
References
Al Dahdah, Marine. 2019. ‘Between Philanthropy and Big Business: The Rise of mHealth in the Global Health Market’. Development and Change 0 (0): 1–20. https://doi.org/10.1111/dech.12497.
Aronowitz, Robert A. 2009. ‘The Converged Experience of Risk and Disease’. The Milbank Quarterly 87 (2): 417–42. https://doi.org/10.1111/j.1468-0009.2009.00563.x
Aronowitz, Robert A. 2015. Risky Medicine: Our Quest to Cure Fear and Uncertainty. Chicago, IL: University of Chicago Press.
Barquera, Simón, Vı́ctor Tovar-Guzmán, Ismael Campos-Nonato, Clicerio González-Villalpando, and Juan Rivera-Dommarco. 2003. ‘Geography of Diabetes Mellitus Mortality in Mexico: An Epidemiologic Transition Analysis’. Archives of Medical Research 34 (5): 407–14. https://doi.org/10.1016/S0188-4409(03)00075-4.
Betancourt, Miguel. 2018. ‘Genómica y Enfermedades Crónicas [Genomics and Chronic Disease]’. Filmed January 29, 2018. Primera Sesión Académica de la Maestría en Salud Pública, Universidad Popular Autónoma del Estado de Puebla, Puebla, México. Video, 1:11:24. https://www.facebook.com/posgradosupaep/videos/1708053432573911.
Birn, Anne-Emanuelle. 2014. ‘Philanthrocapitalism, Past and Present: The Rockefeller Foundation, the Gates Foundation, and the Setting(s) of the International/Global Health Agenda’. Hypothesis 12 (1): 1–27. https://doi.org/0.5779/hypothesis.v12i1.229.
Bishop, Matthew, and Michael Green. 2008. Philanthro-Capitalism: How the Rich Can Save the World. New York, NY: Bloomsbury Press.
Braine, Theresa. 2007. ‘Mexican Billionaire Invests Millions in Latin American Health’. Bulletin of the World Health Organization 85: 574–75. https://doi.org/10.2471/BLT.07.010807.
Broad Institute. 2010. ‘Mexico–US Collaboration Launched’. https://www.broadinstitute.org/news/mexico-us-collaboration-launched.
Broad Institute. n.d. ‘Carlos Slim Center for Health Research at the Broad Institute’. https://www.broadinstitute.org/sigma.
Broadband Comission. 2018. The Promise of Digital Health: Addressing Non-Communicable Diseases to Accelerate Universal Health Coverage in LMICs. Basel, Switzerland: Novartis Foundation.https://www.novartisfoundation.org/sites/arctic_novartisfoundation/files/2020-11/2018-the-promise-of-digital-health-full-report.pdf.
Business Wire. 2014. ‘Metabolon Enters into Agreement with the Carlos Slim Institute, Patia and Clinica Ruiz for Quantose Prediabetes Test in Mexico’. http://www.businesswire.com/news/home/20140224006691/en/Metabolon-Enters-Agreement-Carlos-Slim-Institute-Patia.
Clarke, Adele E., Laura Mamo, Jennifer Ruth Fosket, Jennifer R. Fishman, and Janet K. Shim, eds. 2010. Biomedicalization: Technoscience, Health, and Illness in the US. Durham, NC: Duke University Press.
Clarke, Adele E., Janet K. Shim, Laura Mamo, Jennifer Ruth Fosket, and Jennifer R. Fishman. 2003. ‘Biomedicalization: Technoscientific Transformations of Health, Illness, and US Biomedicine’. American Sociological Review 68 (2): 161–94. https://doi.org/10.2307/1519765.
Cobb, Jeff, Walter Gall, Klaus-Peter Adam, Pamela Nakhle, Eric Button, James Hathorn, Kay Lawton, et al. 2013. ‘A Novel Fasting Blood Test for Insulin Resistance and Prediabetes’. Journal of Diabetes Science and Technology 7 (1): 100–10. https://doi.org/10.1177/193229681300700112.
Cruz Martínez, Ángeles. 2013. ‘Informan sobre prueba específica de predisposición a cáncer de mama. [A specific test for breast cancer predisposition is presented]’. La Jornada, September 25, 2013.http://www.jornada.unam.mx/2013/09/25/sociedad/038n3soc.
Dumit, Joseph. 2012. Drugs for Life: How Pharmaceutical Companies Define our Health. Durham, NC: Duke University Press.
Fejerskov, Adam Moe. 2017. ‘The New Technopolitics of Development and the Global South as a Laboratory of Technological Experimentation’. Science, Technology, & Human Values 42 (5): 947–68. https://doi.org/10.1177/0162243917709934.
Fitipaldi, Hugo, Mark I. McCarthy, Jose C. Florez, and Paul W. Franks. 2018. ‘A Global Overview of Precision Medicine in Type 2 Diabetes’. Diabetes 67 (10): 1911. https://doi.org/10.2337/dbi17-0045.
Fosket, Jennifer. 2010. ‘Breast Cancer Risk as Disease: Biomedicalizing Risk’. In Biomedicalization: Technoscience, Health, and Illness in the US, edited by Adele E. Clarke, Laura Mamo, Jennifer Ruth Fosket, Jennifer R. Fishman, and Janet K. Shim, 331–52. Durham, NC: Duke University Press.
Galván Ochoa, Enrique. 2018. ‘Dinero: Slim cumple 78 años; Sanbornomics y el salario constitutional; Pronostican dólar a 25 pesos’. La Jornada, January 26, 2018. https://www.jornada.com.mx/2018/01/26/opinion/006o1eco.
Greene, Jeremy A. 2007. Prescribing by Numbers: Drugs and the Definition of Disease. Baltimore, MD: Johns Hopkins University Press.
Harris, Paul. 2010. ‘Carlos Slim Helu: In the money, But Who Would Know it?’ The Guardian, March 13, 2010.https://www.theguardian.com/theobserver/2010/mar/14/carlos-slim-helu-rich-bill-gates.
Hess, David J. 2015. ‘Undone Science and Social Movements: A Review and Typology’. In Routledge International Handbook of Ignorance Studies, edited by Matthias Gross and Linsey McGoey, 141–54. Abingdon, UK: Routledge.
Lee, Sandra Soo-Jin. 2017. ‘Consuming DNA: The Good Citizen in the Age of Precision Medicine’. Annual Review of Anthropology 46 (1): 33–48. https://doi.org/10.1146/annurev-anthro-102116-041547.
Mahajan, Manjari. 2018. ‘Philanthropy and the Nation-State in Global Health: The Gates Foundation in India’. Global Public Health 13 (10): 1357–68. https://doi.org.10.1080/17441692.2017.1414286.
Mahajan, Manjari. 2019. ‘The IHME in the Shifting Landscape of Global Health Metrics’. Global Policy 10 (S1): 110–20. https://doi.org/10.1111/1758-5899.12605.
Metabolon. 2017a. ‘About Metabolomics’. http://www.metabolon.com/what-we-do/about-metabolomics.
Metabolon. 2017b. ‘Quantose IR’. http://www.metabolon.com/who-we-serve/clinical-consumer-applications/quantose-ir.
McGoey, Linsey. 2015. No Such Thing as a Free Gift: The Gates Foundation and the Price of Philanthropy. London, UK: Verso.
McGoey, Linsey, and Darren Thiel. 2018. ‘Charismatic Violence and the Sanctification of the Super-Rich’. Economy and Society 47 (1): 111–34. https://doi.org/10.1080/03085147.2018.1448543.
Nelson, Alondra, and Joan H. Robinson. 2014. ‘The Social Life of DTC Genetics: The Case of 23andMe’. In Routledge Handbook of Science, Technology and Society, edited by Daniel Lee Kleinman and Kelly Moore, 108–23. New York, NY: Routledge.
Osorno, Diego Enrique. 2015. Slim: Biografía política del Mexicano más rico del mundo Mexico City, Mexico: Penguin Random House Grupo Editorial.
Parthasarathy, Shobita. 2014. ‘Producing the Consumer of Genetic Testing: The Double-Edged Sword of Empowerment’. In Routledge Handbook of Science, Technology and Society, edited by Daniel Lee Kleinman and Kelly Moore, 93–107. New York, NY: Routledge.
Patia Biopharma. 2015. ‘Quantose RI’. www.patiadiabetes.com/mx/patia/productos/quantose-ri/.
Patia Biopharma. 2014. ‘Desarrollo propio de Patia Biopharma: DIABETESPredict’. http://www.patia.com.mx/diabetes.html (no longer active).
Reubi, David. 2018. ‘Epidemiological Accountability: Philanthropists, Global Health and the Audit of Saving Lives’. Economy and Society 47 (1): 83–110. https://doi.org/10.1080/03085147.2018.1433359.
Rose, Nikolas. 2007. Politics of Life Itself: Biomedicine, Power and Subjectivity in the Twenty-First Century. Princeton, NJ: Princeton University Press.
Rosenberg, Charles. 2009. ‘Managed Fear’. Lancet 373 (9666): 802–03. https://doi.org/10.1016/S0140-6736(09)60467-0.
Ruiz Jaimes, Elizabeth. 2014. ‘Un “gen ahorrador” neandertal, detrás de la epidemia diabética.’ El Economista, February 16, 2014.https://www.eleconomista.com.mx/arteseideas/Un-gen-ahorrador-neandertal-detras-de-la-epidemia-diabetica-20140216-0088.html.
Shiffman, Jeremy. 2014. ‘Knowledge, Moral Claims and the Exercise of Power in Global Health’. International Journal of Health Policy and Management 3 (6): 297–99. https://doi.org/10.15171/ijhpm.2014.120.
Tapia-Conyer, Roberto. 2011. Trends in Global Health: A Social Investment Perspective. Filmed January 25, 2011. University of California Irvine, Irvine, CA: University of California Television. Video, 50:44. https://www.youtube.com/watch?v=-NqGnGMmVSE.
Tapia-Conyer, Roberto. 2014. La revolución digital y genómica para una mejor salud pública. [The digital revolution and genomics for a better public health].’ Filmed March 12, 2014. Academia Nacional de Medicina de México. Video, 22:30. https://www.youtube.com/watch?v=ZGIieFA1GCU.
Tapia-Conyer, Roberto, Héctor Gallardo-Rincón, and Rodrigo Saucedo-Martinez. 2013. ‘CASALUD: An Innovative Health-Care System to Control and Prevent Non-Communicable Diseases in Mexico’. Perspectives in Public Health 135 (4): 180–90. https://doi.org/10.1177/1757913913511423.
Tapia-Conyer, Roberto, Rodrigo Saucedo-Martínez, Ricardo Mújica-Rosales, Héctor Gallardo-Rincón, Evan Lee, Craig Waugh, Lucía Guajardo, et al. 2017. ‘A Policy Analysis on the Proactive Prevention of Chronic Disease: Learnings from the Initial Implementation of Integrated Measurement for Early Detection (MIDO)’. International Journal of Health Policy and Management 6 (6): 339–44. https://doi.org/10.15171/ijhpm.2017.18.
Thompson, Ginger. 2006. ‘Prodded by the Left, Mexico’s Richest Man Talks Equity’. The New York Times, June 3, 2006.https://www.nytimes.com/2006/06/03/world/americas/03slim.html.
Timmermans, Stefan, and Mara Buchbinder. 2010. ‘Patients-in-Waiting: Living between Sickness and Health in the Genomics Era’. Journal of Health and Social Behavior 51 (4): 408–23. https://doi.org/10.1177/0022146510386794.
Toche, Nelly, and Manuel Lino. 2014. ‘Localizan con genómica forma curable de diabetes. [A curable type of diabetes is discovered with genomics]’. El Economista, July 22, 2014. https://www.eleconomista.com.mx/arteseideas/Localizan-con-genomica-forma-curable-de-diabetes-20140722-0016.html.
Vasquez, Emily E., and Vivette García Deister. 2019. ‘Mexican Samples, Latino DNA: The Trajectory of a National Genome in Transnational Science’. Engaging Science, Technology, and Society 5: 107–34. https://doi.org/10.17351/ests2019.199.